Spanish researchers have analyzed X-ray crystal structures of seven previously ‘resurrected’ 1 – 4 billion year old thioredoxins, small redox proteins known to be present in all organisms.
Modern proteins exhibit an impressive degree of structural diversity, which has been well characterized. But very little is known about how and when over the course of evolution protein structures arose.
The new study, reported in the journal Structure, reveals a remarkable degree of structural similarity among proteins since life first developed on Earth.
“So far, attempts to understand protein structure evolution have been based on the comparison between structures of modern proteins. This is equivalent to trying to understand the evolution of birds by comparing several living birds. But it is most useful to study fossils so that changes over evolutionary time are apparent. Our approach comes as close as possible to ‘digging up’ fossil protein structures,” said senior author Dr Jose Sanchez-Ruiz from the University of Granada.
In a 2011 study, the scientists constructed a phylogenetic tree of protein sequences by analyzing the amino acid sequences of thioredoxins-proteins found in organisms from the three domains of life, including bacteria, archaea and eukaryotes. Using this phylogenetic tree, they were able to resurrect Precambrian proteins in the laboratory.
In the new study, they analyzed the X-ray crystal structures of these proteins. They found that present-day thioredoxin structures are remarkably similar to those that existed at a time close to the origin of life, even though their amino acid sequences are very different.
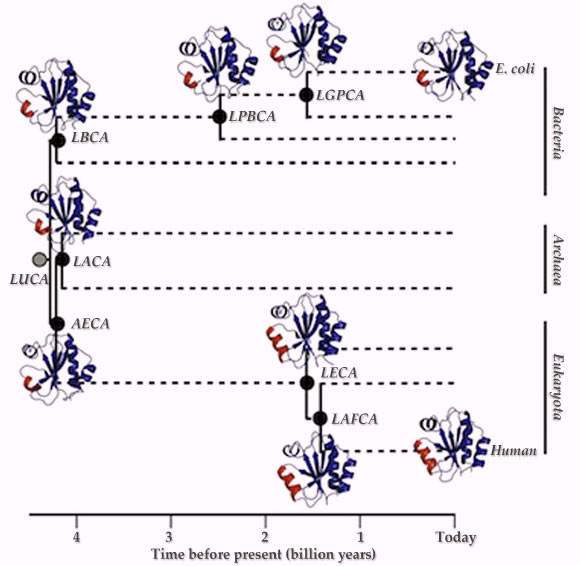
This schematic phylogenetic tree shows the geological time and the phylogenetic nodes targeted in the new study. LBCA – last bacterial common ancestor; LACA – last archaeal common ancestor; AECA – archaeal-eukaryotic common ancestor; LECA – last eukaryotic common ancestor; LAFCA – last common ancestor of fungi and animals; LPBCA – last common ancestor of the cyanobacterial, deinococcus and thermus groups; LGPCA – last common ancestor of g-proteobacteria. LUCA – last universal common ancestor (Ingles-Prieto et al).
This finding supports a punctuated-equilibrium model of evolution in which protein structures remain constant over long time periods, with new changes occurring intermittently over short periods.
“In addition to uncovering the basic principles of protein structure evolution, our approach will provide invaluable information regarding how the 3D structure of a protein is encoded by its amino acid sequence,” Dr Sanchez-Ruiz explained.
“It could also provide information about how to design proteins with novel structures – an important goal in protein engineering and biotechnology.”
______
Bibliographic information: Ingles-Prieto et al. Conservation of Protein Structure over Four Billion Years. Structure, published online August 8, 2013; doi: 10.1016/j.str.2013.06.020