Scientists have discovered a new type of photoreceptor protein that is about 50 times more efficient at capturing light than the rhodopsin, a protein that resides in cell membranes in the retina of the human eye.
The new receptor protein, called LITE-1, was discovered in the nematode worm Caenorhabditis elegans, a common model organism in biological research.
“This photoreceptor actually comes from a family of taste receptor proteins first discovered in insects,” said Shawn Xu, a Professor in the Department of Molecular and Integrative Physiology at the University of Michigan Medical School and senior author of a paper on the discovery published Nov. 17 in the journal Cell.
“These, however, are not the same taste receptors as in mammals.”
Prof. Xu and co-authors previously demonstrated that although they lack eyes, C. elegans will move away from flashes of light.
The new research goes a step further, showing that LITE-1 directly absorbs light, rather than being an intermediary that senses chemicals produced by reactions involving light.
“Several features distinguish LITE-1 from known photoreceptors, including an exceptionally high efficiency in photoabsorption, an ability to sense both UV-A and UV-B light, a strict dependence on protein conformation for photoabsorption, a strong resistance to photobleaching, and a reversed membrane topology compared to opsins,” the authors explained.
“LITE-1 also bears no sequence homology with the two known metazoan photoreceptors (i.e., opsins and cryptochromes) or any other photoreceptors in microbes and plants.”
“Apparently, LITE-1 represents a distinct type of photoreceptor in nature,” they said.
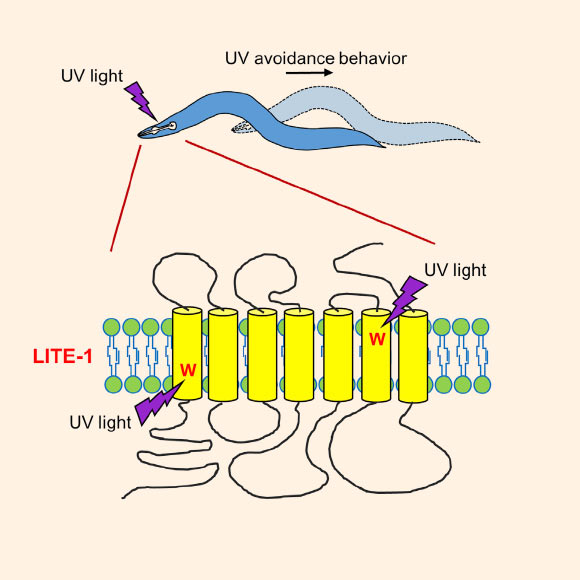
According to the team, LITE-1 absorbs UV light and mediates avoidance behavior in C. elegans in response to light exposure. LITE-1 has unusual characteristics that suggest potential future applications ranging from sunscreen to research tools. Image credit: Jianke Gong et al, doi: 10.1016/j.cell.2016.10.053.
Animal photoreceptors typically have two components: a base protein and a light-absorbing chromophore (a role played by retinal, or vitamin A, in human sight).
When you break these photoreceptors apart, the chromophore still retains some of its functionality.
“This is not the case for LITE-1. Breaking it apart completely stops its ability to absorb light, rather than just diminishing it — showing that it really is a different model,” Prof. Xu said.
The team also determined that within LITE-1, having the amino acid tryptophan in two places was critical to its function.
When a nonlight-sensitive protein in the same family, GUR-3, was modified to add the corresponding tryptophan residues, it reacted strongly to UV light — with about a third the sensitivity to UV-B as LITE-1.
“This suggests scientists may be able to use similar techniques to genetically engineer other new photoreceptors,” Prof. Xu noted.
Characterizing the current research as an ‘entry point,’ the authors said the discovery might prove useful in a variety of ways.
“With further study, for example, it might be possible to develop LITE-1 into a sunscreen additive that absorbs harmful rays, or to further scientific research by fostering light sensitivity in new types of cells,” the researchers said.
_____
Jianke Gong et al. 2016. The C. elegans Taste Receptor Homolog LITE-1 is a Photoreceptor. Cell 167 (5): 1252-1263; doi: 10.1016/j.cell.2016.10.053