An international team of scientists from the United States and China has found a sensor for the reactive molecules linked to diabetic complications. The study, done in the nematode worm Caenorhabditis elegans, provides particular promise for those suffering from painful diabetes-related nerve damage.
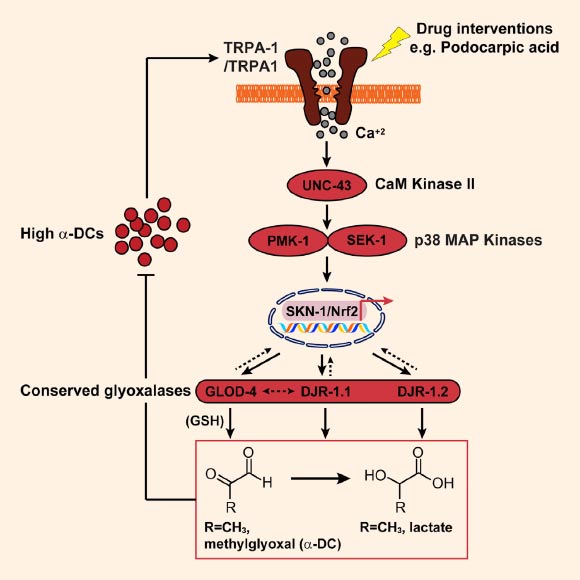
TRPA-1/TRPA1 activation via a-dicarbonyls (a-DCs) is relayed through UNC-43 (CaMKII), PMK-1, and SEK-1 (MAPK) to SKN-1/Nrf, resulting in the expression of various downstream glyoxalases to achieve organism-wide a-DC detoxification. Image credit: Jyotiska Chaudhuri et al, doi: 10.1016/j.cub.2016.09.024.
The team, headed by Prof. Pankaj Kapahi from the Buck Institute for Research on Aging, identified two natural compounds, including the supplement alpha-lipoic acid, which prevented nerve damage in C. elegans experiencing a similar hypersensitivity to touch as do humans who suffer from diabetic neuropathy. The compounds essentially cured the worms of the condition.
“We now have a good model and a novel pathway that allows us to study many of the complications of diabetes,” Prof. Kapahi said.
“We also have novel compounds that slow the accumulation of these toxic molecules and show real promise in alleviating a very painful condition.”
“We realize that it is a huge leap between humans and C. elegans, but it’s important to note that the pathway involved in neuropathy is conserved among species,” he added.
Prof. Kapahi and his colleagues from the Buck Institute, the University of California, San Francisco, the Huazhong University of Science and Technology and the University of Michigan focused on a compound called methylglyoxal (MGO) which is formed from glucose in the body.
MGO is extremely toxic and reacts with essential proteins, DNA, and lipids to yield a heterogeneous group of molecules, collectively called advanced glycation end products (AGEs), which have been implicated as the cause of many diabetic complications.
AGEs are also suspected of contributing to Alzheimer’s and Parkinson’s diseases, which have been linked to type 2 diabetes. AGEs affect nearly every cell type and are a normal byproduct of metabolism and are not generally a problem for those who eat a healthy diet. But the production of AGEs ramps up when blood sugar is out of control, as it is in diabetes.
“At some point, damage from accumulated AGEs becomes irreversible. Our goal is to stop them from forming in the first place,” Prof. Kapahi noted.
The researchers identified a critical sensor for MGO, a protein called TRPA1 (and a similarly named pathway) that responds to high MGO in the body and detoxifies them.
“TRP (Transient Receptor Potential) ion channels are evolutionarily conserved proteins that function in sensing many stimuli,” said co-first author Dr. Jyotiska Chaudhuri, from the Buck Institute.
“Of them, TRPA1 is a well-known mechanosensory receptor that responds to many noxious stimuli — including pain. Because our model exhibited mechanosensory phenotypes we examined if it played a role in diabetic neuropathy.”
The team boosted the activity of TRPA1 by feeding C. elegans alpha-lipoic acid and podocarpic acid.
Alpha-lipoic acid is an antioxidant naturally found in organ meats such as liver and kidney as well as in yeast, spinach and broccoli. It’s also available as a supplement. Several human studies suggest that this compound helps lower blood sugar levels.
Podocarpic acid is a compound found in the bark of a conifer that grows in Australia and New Zealand.
“The results were dramatic. The worms were no longer hypersensitive to touch, they moved normally, they exhibited no neuronal damage and lived a long healthy life,” said co-first author Dr. Neelanjan Bose, from the Buck Institute and the University of California, San Francisco.
“Our work demonstrates that TRPA1 activity is critical in limiting diabetic complications.”
“The new model, with its focus on TRPA-1, MGO and AGEs, will help researchers understand and deal with the complex pathology of type 2 diabetes and its complications,” Prof. Kapahi said.
“The short-lived worm provides a way to get to the root cause of diabetic complications, and also facilitates rapid drug discovery. It can take decades for symptoms to develop in humans – in the worm we see problems within two weeks.”
“The findings could help hone in on research on substances that are known to activate TRPA-1, such as cinnamon, garlic, and wasabi,” he added.
“There is some evidence that people who eat spicy food are protected against diabetes. Maybe it’s because of TRPA-1.”
The team’s results were published online October 20, 2016 in the journal Current Biology.
The authors will next focus on AGEs and their implications for Alzheimer’s and Parkinson’s diseases. They want to see if they can rescue mice who suffer from conditions increasingly linked to type 2 diabetes.
_____
Jyotiska Chaudhuri et al. A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive α-Dicarbonyl Detoxification. Current Biology, published online October 20, 2016; doi: 10.1016/j.cub.2016.09.024
This article is based on a press-release from the Buck Institute for Research on Aging.







