Named the Swift Ray 1, the real-life Star Trek ‘tricorder’ is a novel point-of-care hyperspectral imaging device that allows the acquisition of medical-grade images through a smartphone. Hyperspectral imaging acquires a multi-dimensional image dataset, called a hypercube, that provides diagnostic information about tissue physiology, morphology, and composition. The Swift Ray 1 device is equipped with near- and long-wave infrared sensors, violet light sources, and visible range LEDs, thereby allowing the simultaneous acquisition of visible light, infrared thermography, and bacterial fluorescence images as a hypercube. The device also integrates into the Swift Skin and Wound app, enabling precise wound area measurement, temperature quantification, and fluorescence area quantification.

The Swift Ray 1 imaging device is a pocket-sized hyperspectral camera that is designed to sit over a smartphone’s camera lens and wirelessly connect to Swift Medical’s Skin and Wound app. Image credit: Ramirez-GarciaLuna et al., doi: 10.3389/fmed.2023.1165281.
“Wound care is one of today’s most expensive and overlooked threats to patients and our overall healthcare system,” said Dr. Robert Fraser, a researcher at Western University and Swift Medical Inc.
“Clinicians need better tools and data to best serve their patients who are unnecessarily suffering.”
The scientists developed a device called the Swift Ray 1 which can be attached to a smartphone and connected to the Swift Skin and Wound software.
This can take medical-grade photographs, infrared thermography images (which measure body heat), and bacterial fluorescence images (which reveal bacteria using violet light).
None of these images would be enough to identify infection alone.
Clinical inspection has low accuracy, as does thermography measuring heat changes caused by inflammation and infection.
Bacterial fluorescence can only look at the surface of a wound, which is naturally contaminated with bacteria, so additional methods are needed to differentiate between contamination and an infected wound.
“Research has demonstrated bacterial imaging helps guide clinicians’ work to remove nonviable tissue, yet it cannot identify infection by itself,” said Dr. Jose Ramirez-GarciaLuna, a researcher at McGill University Health Centre.
“Thermography provides insight into the inflammatory and circulatory changes happening under the skin.”
The scientists sought to combine these modalities to come up with a method which wouldn’t need multiple expensive devices, would overcome the weaknesses of each imaging method, and could provide an objective measure of wound healing.
To test their device, they recruited 66 wounded patients.
The patients’ wounds showed no sign of infection spreading further, did not contain foreign bodies, and had not previously been treated with antibiotics or growth factors.
The wounds were uncovered, cleaned, and dried before imaging, and afterwards cared for as usual.
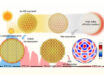
The images were reviewed by a researcher who wasn’t present for the wound care process. Four patterns were identified.
Wounds where the wound was not warmer than healthy skin and no bacterial fluorescence was present were considered ‘non-inflamed,’ while wounds that were slightly warmer than healthy skin and had no or slight bacterial fluorescence were considered ‘inflamed.’
The last two patterns — wounds that were substantially warmer, with or without bacterial fluorescence — were both designated as ‘infected’, because all the clinicians who had examined these wounds had considered them infected.
Out of the 66 wounds, 20 were considered non-inflamed, 26 were inflamed, and 20 were infected.
The researchers performed principal component analysis and used an algorithm called nearest k-neighbor clustering to see if a machine learning model could accurately identify these different categories of wound.
They found that the model could identify all three very well, with an overall accuracy of 74%.
When differentiating between infected vs. non-infected wounds, the model correctly identified 100% of infected wounds and 91% of non-infected wounds.
“This was a pilot study and follow up studies are planned,” Dr. Fraser said.
“In the future, patient populations with more wound types are required to validate across populations.”
The team’s device is described in a paper in the journal Frontiers in Medicine.
_____
Jose L. Ramirez-GarciaLuna et al. 2023. Is my wound infected? A study on the use of hyperspectral imaging to assess wound infection. Front. Med 10; doi: 10.3389/fmed.2023.1165281