Insulating materials can in principle be made metallic by applying pressure. In the case of pure water, this is estimated to require a pressure of 48 Mbars (roughly 48 million times the atmospheric pressure), which is beyond current experimental capabilities and may only exist in the interior of large planets or stars. In new research, chemists from the Institute of Organic Chemistry and Biochemistry at the Czech Academy of Sciences and elsewhere found that a metallic water solution can be prepared by massive doping with electrons upon reacting water with alkali metals.

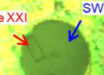
A pure NaK drop exposed to water vapor in vacuum. Image credit: Mason et al., doi: 10.1038/s41586-021-03646-5.
“Throwing sodium into water is one of the most popular school experiments and the subject of many YouTube videos,” said senior author Professor Pavel Jungwirth, a researcher at the Institute of Organic Chemistry and Biochemistry at the Czech Academy of Sciences.
“As is well known, when you throw a chunk of sodium in water, you don’t get metallic water but an immediate and substantial explosion that takes out your apparatus.”
“In order to contain this intense and, for laboratory purposes, rather counterproductive chemistry, we approached it the other way around; instead of adding the alkali metal to the water, we added the water to the metal.”
Professor Jungwirth and his colleagues from Czech Republic, the United States, Germany, Egypt and Japan exposed a drop of sodium-potassium (NaK) alloy to a small amount of water vapor, which began to condense on its surface.
“Our experiment was realized with a NaK alloy that is liquid at room temperature, dripping at a rate of about one drop every 10 s from a micronozzle into a vacuum chamber with a background water vapor pressure that is tunable up to a fraction of a millibar,” they explained.
“With no water vapor in the vacuum chamber, the NaK drops have only a silver metallic sheen. The lack of visible color is due to alkali metals possessing neither d nor f electrons that are subject to optical excitations.”
“When the water vapor pressure in the vacuum chamber is increased to 0.0001 millibar, a sufficient amount of water adsorbs to the surface of the newly formed NaK drops such that their surface layer turns almost immediately gold in color.”
“The golden color persists for up to 5 s, after which, as water continues to adsorb, the color gradually turns bronze for another 2-3 s.”
“Eventually, the drop loses its metallic sheen and turns purple/blue and finally white — the latter due to the formation of an alkali hydroxide layer as a product of the reaction between the metal and water.”
“The whole process lasts for about 10 s, within which the drop grows and reaches its final size of 5 mm in diameter. It then falls off the end of the capillary owing to gravitational force, with a new drop starting to evolve immediately afterwards.”
The researchers confirmed the presence of metallic water using X-ray photoelectron spectroscopy.
“Our study not only shows that metallic water can indeed be produced on Earth, but also characterizes the spectroscopic properties associated with its beautiful golden metallic luster,” said co-author Dr. Robert Seidel, a researcher in the Helmholtz-Zentrum Berlin für Materialien und Energie and the Department of Chemistry at the Humboldt-Universität zu Berlin.
A paper describing the research was published in the July 28, 2021 issue of the journal Nature.
_____
P.E. Mason et al. 2021. Spectroscopic evidence for a gold-coloured metallic water solution. Nature 595, 673-676; doi: 10.1038/s41586-021-03646-5




![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)
