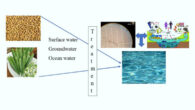
In a new paper published in the journal ChemSystemsChem, Hiroshima University’s Professor Tony Jia and colleagues outline the ‘prebiotic gel-first’ framework, which considers how the origin of life could have potentially emerged within surface-attached gels. The authors also discuss the potential existence of ‘xeno-films,’ i.e., alien biofilm-like structures composed of non-terrestrial — or with some terrestrial — building blocks,...



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-195x110.jpg)