Ice at minus 10 degrees Celsius releases more iron from common minerals than liquid water at 4 degrees Celsius, according to a team of researchers from Umeå University, the Institut des Sciences Chimiques de Rennes and CNRS. This discovery could help explain why many Arctic rivers are now turning rusty orange as permafrost thaws in a warming climate.
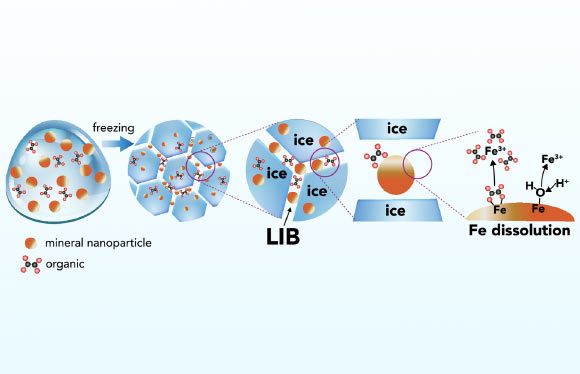
Schematic representation of iron mineral dissolution reactions in ice. Image credit: Sebaaly et al., doi: 10.1073/pnas.2507588122.
“It may sound counterintuitive, but ice is not a passive frozen block,” said Umeå University’s Professor Jean-François Boily.
“Freezing creates microscopic pockets of liquid water between ice crystals.”
“These act like chemical reactors, where compounds become concentrated and extremely acidic.”
“This means they can react with iron minerals even at temperatures as low as minus 30 degrees Celsius.”
To understand the process, Professor Boily and his colleagues studied goethite — a widespread iron oxide mineral — together with a naturally occurring organic acid.
Using advanced microscopy and experiments, they discovered that repeated freeze-thaw cycles make iron dissolve more efficiently.
As the ice freezes and thaws, organic compounds that were previously trapped in the ice are released, fuelling further chemical reactions.
Salinity also plays a crucial role: fresh and brackish water increase dissolution, while seawater can suppress it.
The findings apply mainly to acidic environments, such as mine drainage sites, frozen dust in the atmosphere, acid sulfate soils along the Baltic Sea coast, or in any acidic frozen environment where iron minerals interact with organics.
“As the climate warms, freeze-thaw cycles become more frequent,” said Angelo Pio Sebaaly, a doctoral student at Umeå University.
“Each cycle releases iron from soils and permafrost into the water. This can affect water quality and aquatic ecosystems across vast areas.”
“The findings show that ice is not a passive storage medium, but an active player.”
“As freezing and thawing increase in polar and mountain regions, for the impact on ecosystems and the natural cycling of elements could be significant.”
The team’s paper was published on August 26, 2025 in the Proceedings of the National Academy of Sciences.
_____
Angelo P. Sebaaly et al. 2025. Ice as a kinetic and mechanistic driver of oxalate-promoted iron oxyhydroxide dissolution. PNAS 122 (35): e2507588122; doi: 10.1073/pnas.2507588122