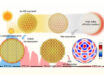
The smallest droplet of water in which ice can form is only as big as 90 water molecules, according to new research.

Moberg et al show that the smallest water clusters that can sustain ice contain only around 90 water molecules. Image credit: Gerd Altmann.
Ice exists on Earth almost exclusively in the highly ordered hexagonal crystal structure known as ice I.
In the atmosphere, small water clusters form and subsequently freeze, seeding larger crystals and eventually clouds. Due to competing thermodynamic effects, however, below a certain diameter these water clusters cannot form thermodynamically stable ice I.
The exact size range of water clusters capable of forming stable ice I has been investigated through experiment and theory for years with most recent estimates narrowing the range from as low as 90 water molecules to as high as 400.
In the past, a major barrier in experimentally studying this limit has been cooling the supercooled liquid clusters slow enough to allow the ice I lattice to form properly.
Cooling too quickly creates clusters of amorphous ice, a less ordered phase. If the clusters are not cooled slowly and uniformly, the result is an unnatural combination of ice phases.
Computer simulations of ice formation also face their own challenges in replicating nanoscale physics and ice formation.
In a new study, an international team of researchers combined recent advances in simulation and experiment to disentangle the interplay between the constraints that act on the ice-liquid transition in nanometer-sized clusters.
To overcome the cooling problem, they used a molecular beam that generates clusters of a desired size by initially expanding a mixture of water and argon through a roughly 60 micrometer diameter nozzle.
The resulting beam is then funneled through three distinct zones where the cooling rate is dropped in order to control the formation of the clusters, reaching a low temperature of 150 K (minus 123 degrees Celsius, or minus 189 degrees Fahrenheit).
Computer models of water developed by the team were used to simulate the properties of the nanodroplets.
Using infrared spectroscopic signatures to monitor the transition to ice I in the clusters, the study authors found promising agreement between the experimental and theoretical approaches.
The results provide strong evidence that the ‘end of ice’ occurs when clusters are around 90 water molecules.
At this size, the clusters are only around 2 nanometers in diameter, or roughly one million times smaller than a typical snowflake.
“This work connects in a consistent manner experimental and theoretical concepts for studying microscopic water properties of the past three decades, which now can be seen in a common perspective,” said Professor Francesco Paesani, from the University of California, San Diego.
Unexpectedly, the scientists found in both simulation and experiment that the coexistence of ice behaves differently in clusters from 90 to 150 water molecules from the sharp, well-defined melting transition we experience with macroscopic (large-scale) ice and water occurring at 0 degrees Celsius.
The clusters were found to instead transition over a range of temperatures and oscillate in time between the liquid and ice states, an effect of their small size that was first predicted three decades ago, but lacked experimental evidence until now.
“Macroscopic systems have no analogous mechanism; water is either liquid or solid. This oscillating behavior seems unique to clusters in this size and temperature range,” said Professor Thomas Zeuch, from the Universität Göttingen.
“There is nothing like these oscillations in our experience of phase coexistence in the macroscopic world,” said Professor Valeria Molinero, from the University of Utah.
The study is published in the Proceedings of the National Academy of Sciences.
_____
Daniel R. Moberg et al. The end of ice I. PNAS, published online November 4, 2019; doi: 10.1073/pnas.1914254116