After decades of groundbreaking laboratory work, the world’s scientific and technical community came together on November 16, 2018, to redefine kilogram (kg), ampere (A), kelvin (K) and mole (mol) — four of the seven base units for the International System of Units (SI), informally known as the metric system. The event was the 26th General Conference of Weights and Measures and was hosted by the International Bureau of Weights and Measures in Versailles, France. The new definitions will be effective on May 20, 2019, World Metrology Day, which celebrates the establishment of the SI in 1875.
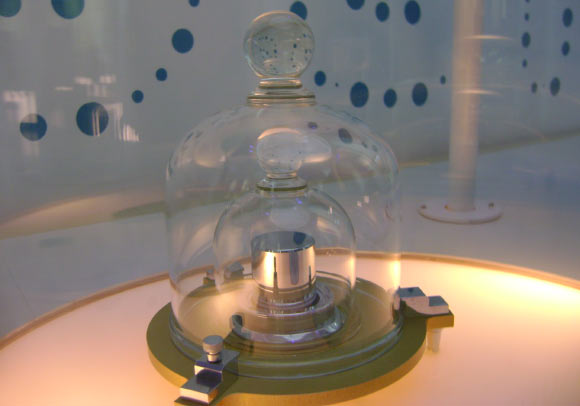
A replica of the prototype of the kilogram at the Cité des Sciences et de l’Industrie in Paris, France. Image credit: Japs / CC BY-SA 3.0.
Currently, there is only one true kilogram, known as Le Grand K, which is secured in a vault outside Paris and used to calibrate all measures of mass throughout the world.
Made of platinum-iridium, Le Grand K, like any alloy, may change over time by absorbing molecules from the air or losing them through cleaning.
However, even these incredibly tiny changes mean that the artifact is no longer accurate enough for anticipated future advanced research and technological applications.
Over the last four decades, with the advancement of quantum science, scientists have measured natural constants such as the speed of light and the Planck constant with exceptional accuracy.
Using combinations of these constants and the equations of quantum mechanics, they created revised SI units for measuring mass, electric current, temperature and the mole that are at least one million times more stable than artifacts like Le Grand K.

This card displays the fundamental constants and other physical values that will define a revised International System of Units. Image credit: Stoughton / NIST.
Scientists have dreamed of having an accurate and precise measurement system that could be realized anytime, anywhere, since the 1700s.
Quantum phenomena that are identical everywhere are already used to define the second, which is the SI unit for time, and the meter, the SI unit for distance.
The second is defined as 9,192,631,770 natural oscillations of microwave radiation released by the element cesium and the meter is defined as the distance traveled by light in vacuum in 1/299,792,458th of a second.
These revised definitions, implemented in 1967 and 1983, respectively, were necessary for the invention of GPS and many other modern technologies.
In May 2019 when the revised definition of the kilogram is implemented, it will be based on three fundamental constants: the Planck constant, the speed of light and the cesium atom’s natural microwave radiation.
The Planck constant describes the size of the packets of energy or quanta that atoms and other particles use to absorb and emit energy.
The current kilogram mass exerts a specific amount of force in Earth’s gravity. The revised definition replaces this determination of mechanical force with an electromagnetic measurement tied to the Planck constant and based on electrical current and voltage.
Using an instrument called a Kibble balance, after its inventor Bryan Kibble, an electric current is generated in a coil to produce a magnetic field strong enough to balance a mass of one kilogram.
The method requires a precision measurement of local gravity, which varies depending on elevation and several other factors. It also requires moving the coil through a magnetic field of known strength and at a known speed, hence the tie as well to constants used to determine time and frequency.
In a similar way, the SI unit for the ampere will now be based on the constant for the charge of the electron.
The kelvin will be based on quantum-level measures of atomic motion and will be tied to the Boltzmann constant that relates an object’s energy to its temperature, as well as on the Planck and cesium frequency constants; while the mole will be based on an improved value for Avogadro’s constant.