Plutonium, a heavy, silvery metal with 15 isotopes that is produced by the neutron irradiation of natural uranium, is formidably complex element that does not always act as expected. New research published in the journal Physical Review B shows that plutonium does not share electrons when it bonds with fluorine atoms.

This image shows a pellet of plutonium-238 dioxide. The pellet is glowing red because of the heat generated by the radioactive decay (primarily alpha) of the fuel. Such pellets were used in radioisotope thermoelectric generators that powered NASA’s Galilleo and Cassini spacecraft on missions to Jupiter and Saturn, respectively. Image credit: Los Alamos National Laboratory.
Plutonium is a transuranic radioactive chemical element with symbol Pu and atomic number 94.
It is an incredibly complex element that has far-ranging energy, security, and environmental effects.
To learn more about plutonium, lead author Dr. Herman Cho of Pacific Northwest National Laboratory and his colleagues delved into a compound with a relatively simple composition: plutonium tetrafluoride (PuF4).
While the formula is simple, the four bonds proved to be more complex. The electrons stay relatively close to each atom, creating ionic bonds — not the expected electron-sharing covalent bonds.
Even though the plutonium and fluorine atoms are tied together in a lattice, they act as isolated atoms and form ionic bonds.
“Bonding is one of the big questions for plutonium and its actinide neighbors on the Periodic Chart,” Dr. Cho said.
“Answering this question is of huge importance because plutonium’s chemistry depends on how it bonds. PuF4 leans toward electrostatic attraction. This work provides a clearer picture of why that is.”
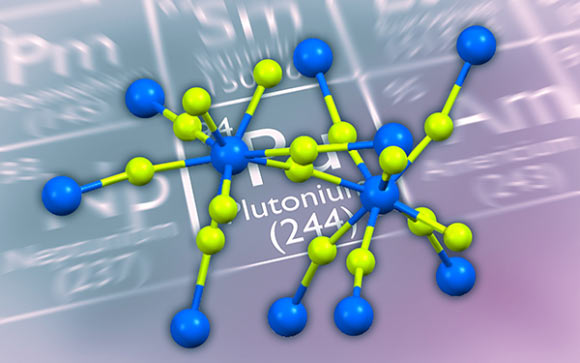
Structure of plutonium tetrafluoride (PuF4): Pu – blue, fluorine – green. Image credit: Cigdem Capan et al.
The scientists began with highly radioactive PuF4 from the long-shuttered Plutonium Finishing Plant in Washington State. At the plant, they created hockey-puck sized ‘buttons’ of plutonium.
They analyzed the plutonium using nuclear magnetic resonance spectroscopy (NMR), which elucidates key features of electronic structure near plutonium centers.
The team examined the atoms in the PuF4. Specifically, the researchers probed the fluorine atoms around the plutonium centers to measure the magnetic fields produced by plutonium (Pu4+), which revealed how the electrons were distributed in the sample.
They determined that the plutonium and fluorine atoms aren’t particularly generous.
Both atoms tend to hold their electrons, acting more like ions in a salt where electrostatic forces hold the atoms together.
This research brings scientists closer to understanding the nuances of plutonium and other actinides, other radioactive elements near the bottom of the Periodic Table.
_____
Cigdem Capan et al. 2016. Probing the Pu4+ magnetic moment in PuF4 with F19 NMR spectroscopy. Phys. Rev. B 93 (22): 224409; doi: 10.1103/PhysRevB.93.224409