Biologists at Vanderbilt University have discovered DNA of the black widow spider toxin in the genome of a virus that attacks Wolbachia, a type of symbiotic bacteria that infects a wide range of invertebrate species, like shrimps, spiders and parasitic worms.
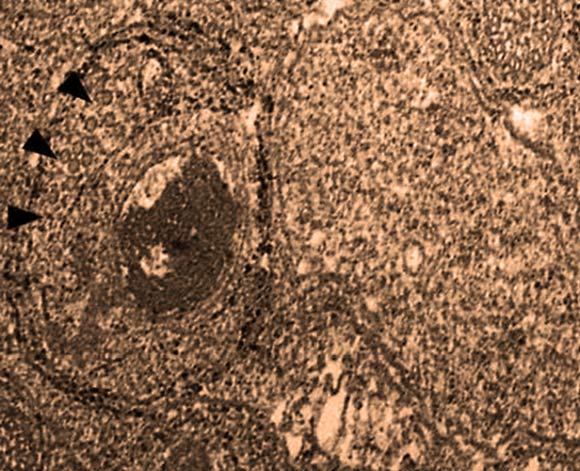
Electron microphotograph shows two Wolbachia cells that have infected a Nasonia wasp. The one to the left is infected with the WO phage, pointed out by the black arrowheads. The one to the right is uninfected. The large dark patch is bacteria DNA that has been degraded by the phage. The image also shows the multiple wasp membranes wrapped around the Wolbachia that the phage must penetrate both to enter and escape from the bacteria. Image credit: Bordenstein Lab, Vanderbilt University.
“Discovering DNA related to the black widow spider toxin gene came as a total surprise because it is the first time that a bacteriophage (phage) – a virus that infects and replicates within bacteria – has been found carrying animal-like DNA,” said Dr. Seth Bordenstein, a biologist in the Department of Biological Sciences and in the Department of Pathology, Microbiology, and Immunology at Vanderbilt University.
Normally, phages — like the WO phage that he and his colleague, Sarah Bordenstein, studied — carry specialized genes that break open and defeat the defenses of the prokaryotic bacterial cells they target.
“But in this case, the portion of DNA related to the black widow spider toxin gene is intact and widespread in the phage,” the scientists said.
“There is also evidence that the phage makes insecticidal toxins, but we are not certain yet how these are utilized and administered.”
They also found that WO shares a number of other segments of DNA with animal genomes. These include a sequence that the eukaryotic cells found in animals use to sense pathogens, which is also involved in triggering cell death. In addition, there were several genes that the cells use to evade immune responses.
“We report a metagenomic analysis of purified phage WO particles of Wolbachia and uncover a eukaryotic association module in the complete WO genome,” the researchers said.
“It harbors predicted domains, such as the black widow latrotoxin C-terminal domain, that are uninterrupted in phage genomes, enriched with eukaryotic protease cleavage sites and combined with additional domains to forge one of the largest phage genes to date.”
“To the best of our knowledge, these eukaryotic-like domains have never before been reported in packaged phages and their phylogeny, distribution and sequence diversity imply lateral transfers between phage/prophage and animal genomes.”
Dr. Bordenstein speculated that the reason WO is exceptional in this regard is due to the life history of its target.
Once Wolbachia infect a host arthropod, it wraps itself in a layer of the arthropod’s membrane. As a result, the phage has to force its way through these eukaryotic membranes in order to enter or escape.
“We suspect it makes pores in the membranes of the arthropod cells that surround Wolbachia, thereby allowing the phage to overcome both the bacterial and arthropod membranes that surround it. That may be how it uses some of these proteins,” the researcher said.
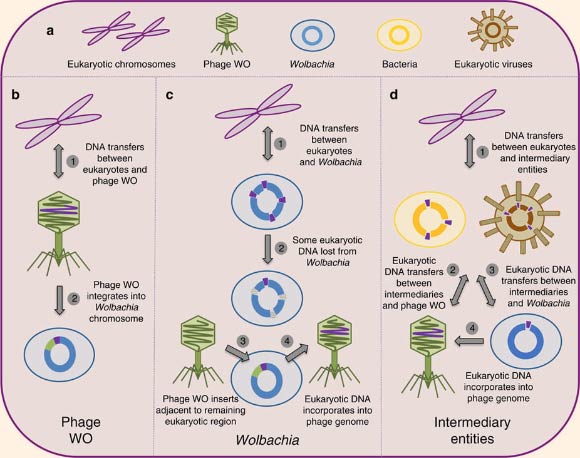
Models of DNA transfer between eukaryotes and phages: (a) the eukaryotic cell can harbor multiple microbes capable of horizontal gene transfer; genetic transfers between eukaryotes and phages can, in theory, occur (b) directly between eukaryotic chromosomes and phage genomes; (c) indirectly between eukaryotic and Wolbachia chromosomes; or (d) indirectly between eukaryotic chromosomes and intermediary entities, such as eukaryotic viruses and other intracellular bacteria. Image credit: Sarah Bordenstein / Seth Bordenstein.
The team also identified the genetic sequences that phage WO uses to insert its genome into the Wolbachia chromosome. This information may provide a basic toolkit that can be used to genetically engineer the bacterium.
This capability could be used to enhance ongoing efforts that use Wolbachia to fight dengue fever and Zika virus.
“The ability to genetically engineer Wolbachia could lead to inserting genes that cause the bacteria to produce traits that increase the effectiveness of using Wolbachia against dengue and Zika viruses,” Dr. Bordenstein said.
“It could also be used to combat other agricultural pests.”
The team’s results were published in the October 11 issue of the journal Nature Communications.
_____
Sarah R. Bordenstein & Seth R. Bordenstein. 2016. Eukaryotic association module in phage WO genomes from Wolbachia. Nature Communications 7, article number: 13155; doi: 10.1038/ncomms13155