Researchers from Germany, French Polynesia and the United Kingdom have successfully sequenced and analyzed the genome of a World Health Organization reference strain of Zika virus.
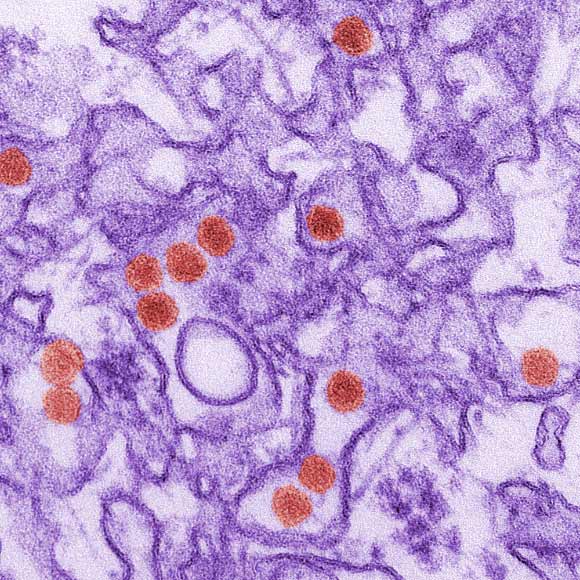
Transmission electron micrograph of Zika virus, which is a member of the family Flaviviridae. Virus particles are 40 nm in diameter, with an outer envelope, and an inner dense core. Image credit: Cynthia Goldsmith / CDC.
Zika virus is a member of the Flavivirus genus in the Flaviviridae family, and nearly 70 years after its discovery in Uganda, it is spreading widely in South and Central America, the Caribbean, and the Pacific.
Infections are frequently asymptomatic or cause mild disease. However, severe complications have recently come to light, including central nervous system abnormalities in fetuses/neonates and Guillain-Barré syndrome (a disorder in which the body’s immune system attacks part of the peripheral nervous system) in adults.
The World Health Organization (WHO) declared the current epidemic a public health emergency of international concern because of these complications.
Dr. Sally Baylis of the Paul-Ehrlich-Institut in Langen, Germany, and co-authors have sequenced a strain of Zika virus that will be used as a WHO reference strain to identify Zika virus infection in the blood.
The strain, called Zika virus strain PF13/251013-18, was isolated from a French Polynesian patient in 2013.
According to the team, the complete sequence of PF13/251013-18 has 10,769 base pairs.
“Reference standards from the WHO are used to harmonize assays for diagnostic testing, particularly in the case of acute infection, as well as assays that might be used to screen blood for transfusions, and to define regulatory requirements for test sensitivity where screening is implemented,” said Dr. Baylis, senior author of a paper reporting the results in the journal Genome Announcements.
While the reference material will undergo formal WHO review in October 2016, the agency has given the go-ahead for the strain’s use given the urgent need of medical products to diagnose and treat Zika.
“WHO’s go-ahead before it’s expert committee meeting in October reflects the urgent need for researchers and companies to access valid reference material to diagnose Zika virus infection,” Dr. Baylis explained.
“This will facilitate the development of sensitive, better performing tests to detect Zika in patients.”
_____
Trösemeier J-H et al. 2016. Genome sequence of a candidate World Health Organization reference strain of Zika virus for nucleic acid testing. Genome Announc. 4 (5): 00917-16; doi: 10.1128/genomeA.00917-16







