When two diatomic molecules collide, they can sometimes swap partners. For instance, two potassium-rubidium (KRb) molecules can produce K2 and Rb2. The four-atom intermediate formed upon collision is typically too scarce and short-lived to spot. Harvard University’s Dr. Kang-Kuen Ni and colleagues circumvented this problem by studying the reaction at a temperature of 500 nanokelvin (just a few millionths of a degree above absolute zero). Using mass spectrometry and velocity-map imaging of a trapped gas of KRb molecules, the researchers directly observed reactants, intermediates, and products of the reaction.
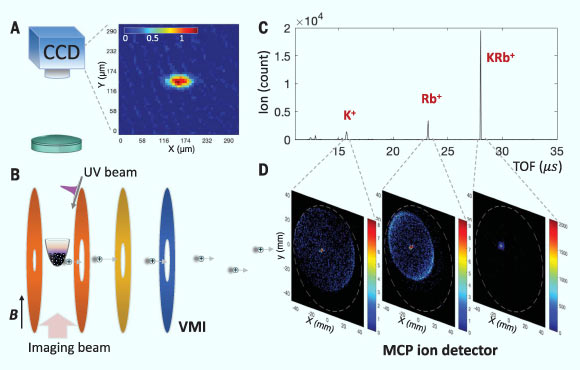
Schematic of the team’s ultracold chemistry apparatus; ground-state KRb molecules at 500 nK are trapped by a crossed optical dipole trap: (A) absorption image of KRb molecules; the color bar indicates the optical depth of the KRb cloud; CCD – charge-coupled device; (B) the trapped molecules are surrounded by velocity-map imaging (VMI) ion optics , which consist of a series of disk-shaped electrodes; the researchers use a pulsed UV laser to photoionize the molecules; B – magnetic field; (C) example time of flight (TOF) spectrum; (D) for each species identified in the mass spectrum, the scientists also obtain a velocity-map image from which the momentum distribution can be inferred. Image credit: Hu et al, doi: 10.1126/science.aay9531.
Chemical reactions are responsible for literally everything: breathing, cooking, digesting, creating energy, pharmaceuticals, and household products like soap. So, understanding how they work at a fundamental level could help researchers design combinations the world has never seen.
With an almost infinite number of combinations possible, new molecules could have endless applications from more efficient energy production to new materials like mold-proof walls and even better building blocks for quantum computers.
In a previous study, Dr. Ni and co-authors used extremely low temperatures to produce molecules from atoms that would otherwise never react.
Cooled to such extremes, atoms and molecules slow to a quantum crawl, their lowest possible energy state.
There, the researchers can manipulate molecular interactions with utmost precision. But even they could only see the start of their reactions: two molecules go in, but then what?
What happened in the middle and the end was a ‘black hole’ only theories could try to explain.
Chemical reactions occur in just millionths of a billionth of a second, better known in the scientific world as femtoseconds.
Even today’s most sophisticated technology can’t capture something so short-lived, though some come close.
In the several past decades, scientists have used ultra-fast lasers like fast-action cameras, snapping rapid images of reactions as they occur. But they can’t capture the whole picture.
“Most of the time you just see that the reactants disappear and the products appear in a time that you can measure,” Dr. Ni explained.
“There was no direct measurement of what actually happened in these chemical reactions — until now.”
The team’s ultracold temperatures force reactions to a comparatively numbed speed.
“Because the molecules are so cold, now we kind of have a bottleneck effect,” Dr. Ni said.
When the scientists reacted two KRb molecules — chosen for their pliability — the ultracold temperatures forced the molecules to linger in the intermediate stage for microseconds.
Microseconds — mere millionths of a second — may seem short, but that’s millions of times longer than usual and long enough for them to investigate the phase when bonds break and form, in essence, how one molecule turns into another.
“With this intimate vision, we can test theories that predict what happens in a reaction’s black hole to confirm if they got it right,” Dr. Ni said.
“Then, we can craft new theories, using actual data to more precisely predict what happens during other chemical reactions, even those that take place in the mysterious quantum realm.”
Already, the chemists are exploring what else they can learn in their ultracold test bed.
Next, for example, they could manipulate the reactants, exciting them before they react to see how their heightened energy impacts the outcome. Or, they could even influence the reaction as it occurs, nudging one molecule or the other.
“With our controllability, this time window is long enough, we can probe,” said Dr. Ming-Guang Hu, a postdoctoral researcher at Harvard University.
“Now, with this apparatus, we can think about this. Without this technique, without this paper, we cannot even think about this.”
The team’s paper was published in the journal Science.
_____
M.-G. Hu et al. 2019. Direct observation of bimolecular reactions of ultracold KRb molecules. Science 366 (6469): 1111-1115; doi: 10.1126/science.aay9531



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)



