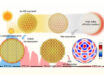
According to an international team of physical chemists, led by University of Luxembourg researcher Prof. Alexandre Tkatchenko, intermolecular attractions known as van der Waals forces have a wave-like nature.
Molecules can attract each other at moderate distances and repel each other at close range. The attractive forces are collectively called van der Waals forces.
Named after Dutch scientist Johannes Diderik van der Waals, these forces are much weaker than chemical bonds, and random thermal motion around room temperature can usually overcome or disrupt them.
“In the simplest case, you can think of two chains of atoms and you could identify points in these chains that are attracted to each other,” Prof. Tkatchenko said.
“Typically, you would compute the van der Waals energy by just summing up all these pairs.”
“However, we demonstrated that at realistic distances between nanoscale materials this is not true.”
Prof. Tkatchenko and his colleagues showed that these interactions have to be treated as coupling between waves rather than as mutual attraction between particles.
“This drastically affects the way we think about these omnipresent interactions,” he said.
The research, published in the journal Science, is likely to have an important impact on material science.
“Over the last two decades, scientists managed to change the properties of existing materials by incorporating nanomaterials, for example they enhanced stress response or achieved high conductivity of polymer composites.”
“In order to understand all the properties of such nanocomposites you have to comprehend how they self-assemble at the nanoscale. The assembly of these materials is mainly driven by van der Waals interactions,” Prof. Tkatchenko said.
As van der Waals forces are critical for many industrial applications, such as the manufacture of nanocomposites, this work could have a great impact on the refinement of processing techniques in that area.
“This work provides both a qualitatively correct conceptual framework for describing van der Waals forces at the nanoscale as well as a quantitatively accurate computational framework for predicting how these ubiquitous interactions influence the physical and chemical properties of matter,” said co-authors Dr. Robert DiStasio Jr., from Cornell University.
_____
Alberto Ambrosetti et al. 2016. Wavelike charge density fluctuations and van der Waals interactions at the nanoscale. Science, vol. 351, no. 6278, pp. 1171-1176; doi: 10.1126/science.aae0509