An international team of scientists, led by Xiao Cheng Zeng from the University of Nebraska-Lincoln and Jijun Zhao from the Dalian University of Technology, has predicted a new molecular form of ice called ice XVII.
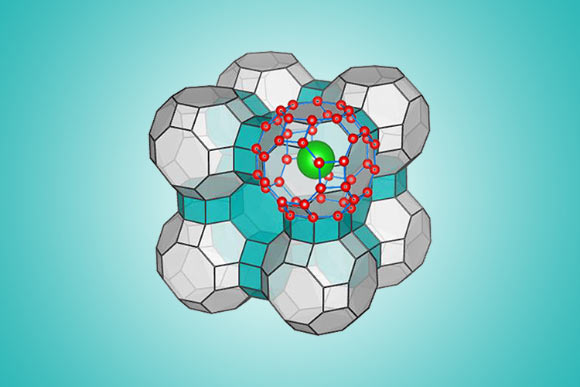
An illustration of the ice XVII’s molecular configuration. Image credit: Yingying Huang / Chongqin Zhu.
If the proposed ice XVII can be synthesized, it would become the 18th known crystalline form of water, according to Prof. Zeng, Dr. Zhao and their colleagues.
It would be about 25% less dense than a record-low form, ice XVI, synthesized by Dr. Werner Kuhs from the University of Göttingen and co-authors in 2014.
“We performed a lot of calculations whether this is not just a low-density ice, but perhaps the lowest-density ice to date. A lot of people are interested in predicting a new ice structure beyond the state of the art,” said Prof. Zeng, who is the senior author of a paper in the journal Science Advances.
Prof. Zeng, Dr. Zhao and their colleagues used a computational algorithm and molecular simulation to determine the ranges of extreme pressure and temperature under which water would freeze into the predicted configuration.
That configuration takes the form of a clathrate — a series of water molecules that form an interlocking cage-like structure.
It was long believed that these cages could maintain their structural integrity only when housing ‘guest molecules’ such as methane, which fills an abundance of natural clathrates found on the ocean floor and in permafrost.
Like Dr. Kuhs’ team before them, however, the chemists have calculated that their clathrate would retain its stability even after its guest molecules have been evicted.
Actually synthesizing the clathrate will take some effort. Based on the team’s calculations, the new ice will form only when water molecules are placed inside an enclosed space that is subjected to ultra-high, outwardly expanding pressure.
“At minus 10 degrees Fahrenheit, the enclosure would need to be surrounded by expansion pressure about four times greater than what is found at the Pacific Ocean’s deepest trench,” the researchers explained.
“At minus 460 degrees Fahrenheit (minus 273 degrees Celsius), that pressure would need to be even greater – roughly the same amount experienced by a person shouldering 300 jumbo jets at sea level.”
“Ice XVII is the most stable ice polymorph in the pressure region below −5834 bar at 0 K (minus 460 degrees Fahrenheit, or minus 273 degrees Celsius) and below −3411 bar at 300 K (80 degrees Fahrenheit, or 29 degrees Celsius),” they wrote in the Science Advances paper.
_____
Yingying Huang et al. 2016. A new phase diagram of water under negative pressure: The rise of the lowest-density clathrate s-III. Science Advances, vol. 2, no. 2, e1501010; doi: 10.1126/sciadv.1501010







