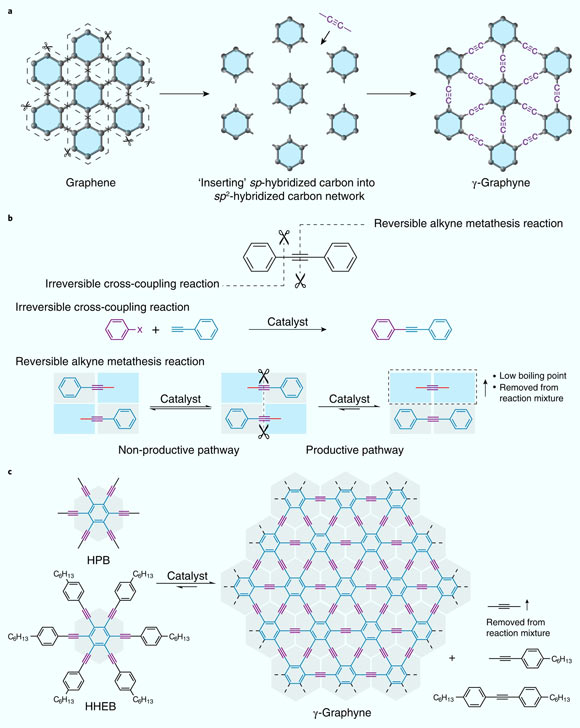
Graphynes are two-dimensional carbon allotropes similar to the wonder material graphene that is optically transparent and mechanically flexible, and yet strong and electronically conductive.
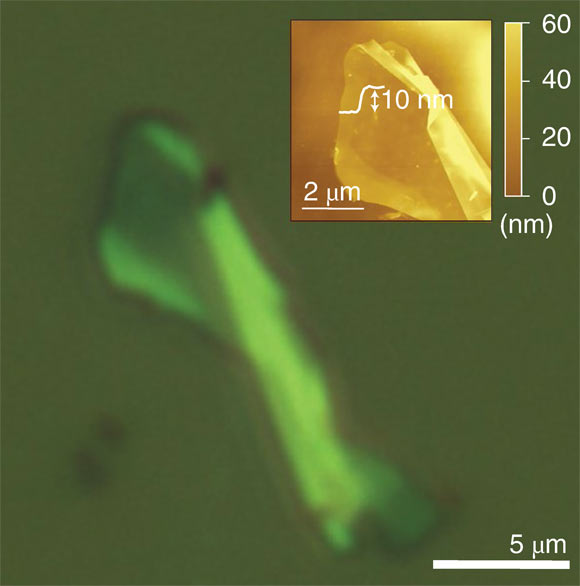
Optical microscopy of gamma-graphyne on 100 nm SiO2/Si; the inset: corresponding AFM image showing the step height. Image credit: Hu et al., doi: 10.1038/s44160-022-00068-7.
Carbon atoms can be sp3–, sp2– or sp-hybridized (different ways they can bind to other elements) to form single, double or even triple bonds with neighboring carbon atoms and so produce various allotropes. The most well-known carbon allotropes are graphite and diamond.
Carbon allotropes have distinct physical properties that arise from the unique combination and arrangement of multiple types of bonds that have various length, strength, geometry and electronic properties.
For instance, graphite is opaque and soft, whereas diamond is transparent and the hardest known natural substance.
Tremendous research efforts have been devoted to constructing new carbon allotropes, which include fullerene (awarded the Nobel Prize in Chemistry in 1996), carbon nanotubes, graphene (awarded Nobel Prize in Physics in 2010), a biphenylene network and cyclo[18]carbon.
All of the above known allotropes are composed of a single type of carbon atom. However, there are many more composed of various combinations of sp3–, sp2– and sp-hybridized carbon atoms and yet to be discovered.
Unlike graphenes, which consist solely of sp2–hybridized carbons, graphynes contain sp-hybridized carbons periodically integrated into an sp2–hybridized carbon framework.
It was predicted that graphyne would exhibit intriguing and unique electron-conducting, mechanical and optical properties.
Specifically, the electron conduction in graphynes would be exceptionally fast, as it is in graphene. Yet, the electron conduction in some graphynes could be controlled in a defined direction, unlike the multidirectional conduction in graphene.
A variety of low-molecular-weight graphyne fragments or ethynylene-linked molecular architectures, which include carbyne, were synthesized by several research groups.
“Creating graphyne is a really old, long-standing question, but since the synthetic tools were limited, the interest went down,” said first author Yiming Hu, a researcher with the Department of Chemistry at the University of Colorado Boulder.
“We brought out the problem again and used a new tool to solve an old problem that is really important.”
Using alkyne metathesis reaction (organic reaction that entails the redistribution, or cutting and reforming, of alkyne chemical bonds), thermodynamics and kinetic control, Hu and colleagues were able to successfully synthesize gamma-graphyne.
“There’s a pretty big difference between graphene and graphyne but in a good way,” said senior author Professor Wei Zhang, also from the Department of Chemistry at the University of Colorado Boulder.
“This could be the next generation wonder material. That’s why people are very excited.”
While the material has been successfully created, the researchers still want to look into the particular details of it, including how to create the material on a large scale and how it can be manipulated.
“We are really trying to explore this novel material from multiple dimensions, both experimentally and theoretically, from atomic-level to real devices,” Professor Zhang said.
“These efforts, in turn, should aid in figuring out how the material’s electron-conducting and optical properties can be used for industry applications like lithium-ion batteries.”
“We hope in the future we can lower the costs and simplify the reaction procedure, and then, hopefully, people can really benefit from our research,” Hu said.
The team’s paper was published in the journal Nature Synthesis.
_____
Y. Hu et al. Synthesis of γ-graphyne using dynamic covalent chemistry. Nat. Synth, published online May 9, 2022; doi: 10.1038/s44160-022-00068-7