Water in a one-molecule layer acts like neither a liquid nor a solid, and becomes highly conductive at high pressures, according to a new paper published in the journal Nature.
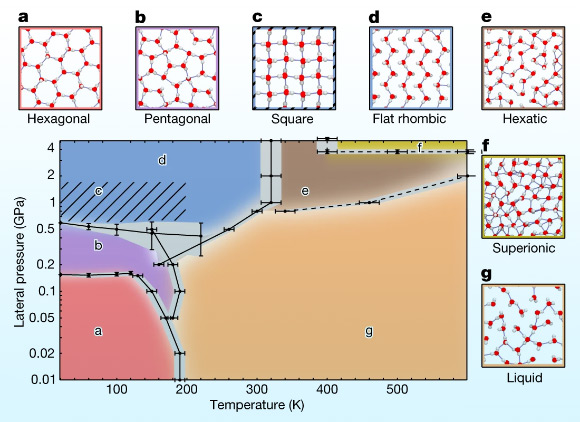
Kapil et al. describe the phase behavior of monolayer-confined water by calculating its pressure-temperature phase diagram: (a-g) the pressure-temperature phase diagram of monolayer water was calculated using an machine learning potential that delivers first-principles accuracy; the solid and dashed lines indicate first-order and continuous phase transitions, respectively; the gray regions indicate the statistical uncertainty for solid-solid phase transitions and, for the other transitions, the uncertainties arising from studying a finite number of thermodynamic states; the diagonally hatched area indicates the region in which square and flat-rhombic phases are near degenerate; diagrams of the hexagonal (a), pentagonal (b), square (c), flat-rhombic (d), hexatic (e), superionic (f) and liquid (g) phases are shown with oxygen atoms in red, hydrogen atoms in gray and hydrogen bonds shown by blue lines. Image credit: Kapil et al., doi: 10.1038/s41586-022-05036-x.
Water trapped between membranes or in tiny nanoscale cavities is common. It can be found in everything from membranes in our bodies to geological formations.
But this monolayer-confined water behaves very differently from the water we drink.
Until now, the challenges of experimentally characterizing the phases of water on the nanoscale have prevented a full understanding of its behavior.
In the new study, University of Cambridge researcher Venkat Kapil and colleagues set out to predict the phase diagram of a one-molecule thick layer of water with unprecedented accuracy.
They used a combination of computational approaches to enable the first-principles level investigation of a single layer of water.
They found that water which is confined into a one-molecule thick layer goes through several phases, including a ‘hexatic’ phase and a ‘superionic’ phase.
In the hexatic phase, the water acts as neither a solid nor a liquid, but something in between.
In the superionic phase, which occurs at higher pressures, the water becomes highly conductive, propelling protons quickly through ice in a way resembling the flow of electrons in a conductor.
“For all of these areas, understanding the behavior of water is the foundational question,” Dr. Kapil said.
“Our approach allows the study of a single layer of water in a graphene-like channel with unprecedented predictive accuracy.”
The researchers found that the one-molecule thick layer of water within the nanochannel showed rich and diverse phase behavior.
Their approach predicts several phases which include the hexatic phase — an intermediate between a solid and a liquid — and also a superionic phase, in which the water has a high electrical conductivity.
“The hexatic phase is neither a solid nor a liquid, but an intermediate, which agrees with previous theories about 2D materials,” Dr. Kapil said.
“Our approach also suggests that this phase can be seen experimentally by confining water in a graphene channel.”
“The existence of the superionic phase at easily accessible conditions is peculiar, as this phase is generally found in extreme conditions like the core of Uranus and Neptune.”
“One way to visualize this phase is that the oxygen atoms form a solid lattice, and protons flow like a liquid through the lattice, like kids running through a maze.”
“This superionic phase could be important for future electrolyte and battery materials as it shows an electrical conductivity 100 to 1,000 times higher than current battery materials.”
“The results will not only help with understanding how water works at the nanoscale, but also suggest that ‘nanoconfinement’ could be a new route into finding superionic behavior of other materials.”
_____
V. Kapil et al. The first-principles phase diagram of monolayer nanoconfined water. Nature, published September 14, 2022; doi: 10.1038/s41586-022-05036-x