Obesity is associated with increased blood concentrations of the anorexigenic hormone leptin; however, obese individuals are resistant to its anorexigenic effects. An international team of researchers has found that mice fed a high-fat diet produce an enzyme named matrix metalloproteinase-2 (MMP-2) that clips receptors for leptin from the surface of neuronal cells in the hypothalamus; this blocks leptin from binding to its receptors; this in turn keeps the neurons from signaling that your stomach is full and you should stop eating. The results, published in the journal Science Translational Medicine, suggest that targeting MMP-2 might be an effective strategy for treating obesity by restoring the anorexigenic effects of leptin.
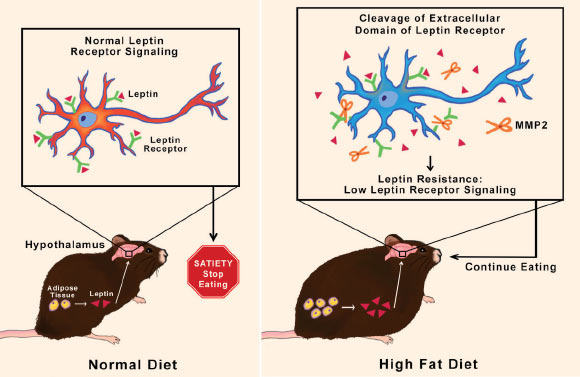
Mazor et al showed that, in rodents, obesity induced matrix MMP-2 activation in the hypothalamus. In turn, MMP-2 activation reduced leptin-mediated signaling by promoting leptin receptor degradation. MMP-2 deletion in the hypothalamus increased leptin receptor expression and reduced fat accumulation in mice fed a high-fat diet. Image credit: Mazor et al, doi: 10.1126/scitranslmed.aah6324.
Leptin molecules are released from white fat tissue during a meal. They travel through the blood stream into the brain, specifically the hypothalamus, where they stimulate neural receptors to signal that the stomach is full. People who are obese often have plenty of leptin in their blood, but it fails to lead to signaling satiety.
Leptin resistance is a known process associated with obesity, but the molecular mechanisms by which it occurs were not understood.
“We opened a new field of study for metabolic disease. We need to ask what other pathways, in addition to leptin and its receptors, undergo a similar destructive process and what the consequences might be,” said co-lead author Dr. Rafi Mazor, a researcher in the Department of Bioengineering at the University of California, San Diego.
While other research efforts have focused on studying pathways that block leptin from doing its job, Dr. Mazor and colleagues decided to investigate the leptin receptor in the brain itself.
“Our hypothesis was that an enzyme breaking down proteins into amino acids and polypeptides can cleave membrane receptors and lead to dysfunctional activity,” Dr. Mazor explained.
“We’re calling for a large-scale clinical trial to investigate whether MMP-2 inhibitors might help people lose weight.”
“Those in the early stages of being overweight might be clipping their leptin receptors, but their neural pathways are still intact,” said study senior author Professor Geert Schmid-Schonbein, also from the University of California, San Diego.
“Receptors are able to regenerate but it’s unclear to what extent. When you block the protease that leads to the receptors not signaling, you can treat the issue.”
Dr. Mazor, Professor Schmid-Schonbein and co-authors first tested brain tissue from obese mice for protease activity. This is how they found MMP-2, the enzyme that they suspected was damaging leptin receptors.
They then developed a method to tag leptin receptors to see what was happening to them. They observed that MMP-2 was damaging the receptors, which lost their ability to signal.
The scientists then used a recombinant protein to verify that the MMP-2 enzyme was indeed cleaving leptin receptors.
They also cultured brain cells from mice and found clipped receptors when MMP-2 was present.
They genetically altered a group of mice to not produce MMP-2. In spite of being fed a high-fat diet, these mice gained less weight and their leptin receptors remained intact. Meanwhile, mice that were fed the same diet but were not genetically altered became obese and their leptin receptors were cleaved.
In the long run, the team aims to design an MMP-2 inhibitor or an inhibitor for the MMP-2 pathway of activation.
Next steps also include confirming that the same mechanism occurs in human brain cells.
“In the future, we will try to find out why proteases are activated, what is activating them and how to stop it,” Dr. Mazor said.
“We think that other membrane receptors may also be destroyed in the same way.”
“There is still a lot of work to do to better understand receptor cleaving and the loss of cell function while on a high-fat diet.”
_____
Rafi Mazor et al. 2018. Cleavage of the leptin receptor by matrix metalloproteinase-2 promotes leptin resistance and obesity in mice. Science Translational Medicine 10 (455): eaah6324; doi: 10.1126/scitranslmed.aah6324







