Curiosity has found evidence that chemistry in the Martian surface material contributed dynamically to the makeup of the planet’s atmosphere over time.

This image from Curiosity’s Mast Camera shows inclined strata at Zabriskie Plateau, about a third of a mile (0.5 km) northeast of the ‘Pahrump Hills’ outcrop at the base of Mount Sharp, the central peak within the Gale Crater on Mars. These sedimentary rocks are inclined toward the south and are also interpreted as the deposits of small deltas building out into a lake. The Mast Camera’s left-eye camera recorded the component frames of this mosaic on July 22, 2014, during the 696th Martian day of Curiosity’s work on Mars. The color has been approximately white-balanced to resemble how the scene would appear under daytime lighting conditions on Earth. Image credit: NASA / JPL-Caltech / MSSS.
The findings, published in the journal Earth and Planetary Science Letters, come from Curiosity’s SAM (Sample Analysis at Mars) instrument suite, which measured all of the stable isotopes of the heavy noble gases krypton and xenon in the Martian atmosphere in situ at Gale Crater.
A lot of information about these gases in the planet’s atmosphere came from analyses of Martian meteorites and measurements made by NASA’s Viking mission.
The new results generally agree with Mars meteorite measurements, but also provide a unique opportunity to identify various non-atmospheric heavy noble gas components in the meteorites.
“What we found is that earlier studies of xenon and krypton only told part of the story,” said lead author Dr. Pamela Conrad, of NASA’s Goddard Space Flight Center.
“SAM is now giving us the first complete in situ benchmark against which to compare meteorite measurements.”
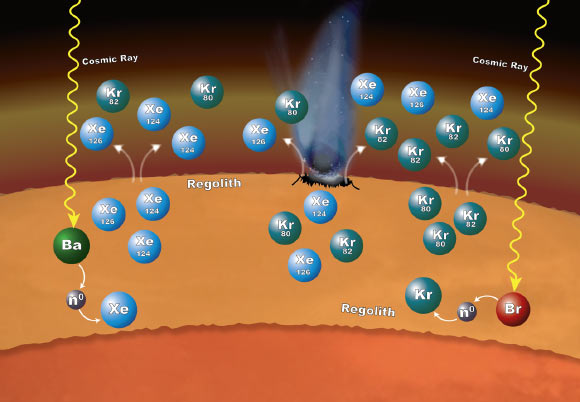
Chemistry that takes place in the Martian surface material can explain why particular xenon (Xe) and krypton (Kr) isotopes are more abundant in the planet’s atmosphere than expected. The isotopes are formed in the loose rocks and material that make up the regolith. The chemistry begins when cosmic rays penetrate into the surface material. If the cosmic rays strike an atom of barium (Ba), the barium can lose one or more of its neutrons (n0). Atoms of xenon can pick up some of those neutrons – a process called neutron capture – to form the isotopes xenon-124 and xenon-126. In the same way, atoms of bromine (Br) can lose some of their neutrons to krypton, leading to the formation of krypton-80 and krypton-82 isotopes. These isotopes can enter the atmosphere when the regolith is disturbed by impacts and abrasion and gas escapes from the regolith. Image credit: NASA’s Goddard Space Flight Center.
The team’s findings agreed with earlier studies, but some isotope ratios were a bit different than expected.
When working on an explanation for those subtle but important differences, the authors realized that neutrons might have gotten transferred from one chemical element to another within the surface material.
The process is called neutron capture, and it would explain why a few selected isotopes were more abundant than previously thought possible.
In particular, it looks as if some of the barium surrendered neutrons that got picked up by xenon to produce higher-than-expected levels of the isotopes xenon-124 and 126.
Likewise, bromine might have surrendered some of its neutrons to produce unusual levels of krypton-80 and krypton-82.
These isotopes could have been released into the atmosphere by impacts on the surface and by gas escaping from the regolith, which is the soil and broken rocks of the surface.
“SAM’s measurements provide evidence of a really interesting process in which the rock and unconsolidated material at the planet’s surface have contributed to the xenon and krypton isotopic composition of the atmosphere in a dynamic way,” Dr. Conrad said.
The atmospheres of Earth and Mars exhibit very different patterns of xenon and krypton isotopes, particularly for xenon-129. Mars has much more of it in the atmosphere than does our planet.
_____
P.G. Conrad et al. 2016. In situ measurement of atmospheric krypton and xenon on Mars with Mars Science Laboratory. Earth and Planetary Science Letters 454: 1-9; doi: 10.1016/j.epsl.2016.08.028
This article is based on a press-release issued by NASA.







