So-called fibrous diamonds, which are cloudy and less appealing to jewelers — and less often, gem-quality diamonds — trap and preserve fluids that are present during their formation. In a series of high-pressure, high-temperature experiments, an international team of geoscientists has demonstrated that seawater in sediments from the bottom of the ocean reacts in the right way to produce the balance of salts found in fibrous diamonds.
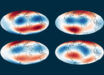
![Backscattered electron images of experimental charges. Locations of images (A) to (D) from sediment-peridotite reaction experiments are schematically shown in capsule on the left. (A, C, and D) - reaction experiments at 5 GPa/1000 degrees Celsius with superimposed energy-dispersive x-ray maps of chlorine [green in (C) and (D)]. The sediment half of two-layer experiments recrystallized to garnet and clinopyroxene (Cpx), whereas orthopyroxene (Opx), magnesite (Mgs), and Na-K chlorides formed at the leading edge of the reaction zone against the peridotite. (B) - peridotite layer in the reaction experiment at 3 GPa/900 degrees Celsius contained phlogopite (Phl) behind the magnesite plus orthopyroxene zone, and Na-K chlorides were absent. (E and F) - sediment melting experiment (no peridotite included) at 4 GPa/1000 degrees Celsius showing silicate melt (E) in equilibrium with garnet (Gt), coesite (Coe), and Mg calcite (Mg-Cc) shown in (F). Scale bars - 100 μm (A and B) and 20 μm (C to F). Image credit: Förster et al, doi: 10.1126/sciadv.aau2620.](https://cdn.sci.news/images/2019/05/image_7241-Diamond-Salts.jpg)
Backscattered electron images of experimental charges. Locations of images (A) to (D) from sediment-peridotite reaction experiments are schematically shown in capsule on the left. (A, C, and D) – reaction experiments at 5 GPa/1000 degrees Celsius with superimposed energy-dispersive x-ray maps of chlorine [green in (C) and (D)]. The sediment half of two-layer experiments recrystallized to garnet and clinopyroxene (Cpx), whereas orthopyroxene (Opx), magnesite (Mgs), and Na-K chlorides formed at the leading edge of the reaction zone against the peridotite. (B) – peridotite layer in the reaction experiment at 3 GPa/900 degrees Celsius contained phlogopite (Phl) behind the magnesite plus orthopyroxene zone, and Na-K chlorides were absent. (E and F) – sediment melting experiment (no peridotite included) at 4 GPa/1000 degrees Celsius showing silicate melt (E) in equilibrium with garnet (Gt), coesite (Coe), and Mg calcite (Mg-Cc) shown in (F). Scale bars – 100 μm (A and B) and 20 μm (C to F). Image credit: Förster et al, doi: 10.1126/sciadv.aau2620.
While gem-quality diamonds are usually made of pure carbon, fibrous diamonds often include small traces of sodium, potassium and other minerals that reveal information about the environment where they formed. These fibrous diamonds are commonly ground down and used in technical applications like drill bits.
“We knew that some sort of salty fluid must be around while the diamonds are growing, and now we have confirmed that marine sediment fits the bill,” said lead author Dr. Michael Förster, from the ARC Centre of Excellence of Core to Crust Fluid Systems and the Department of Earth and Planetary Sciences at Macquarie University.
For this process to occur, a large slab of sea floor would have to slip down to a depth of more than 124 miles (200 km) below the surface quite rapidly, in a process known as subduction in which one tectonic plate slides beneath another.
The rapid descent is required because the sediment must be compressed to more than 4 GPa (gigapascals) — or 40,000 times atmospheric pressure — before it begins to melt in the temperatures of more than 1,472 degrees Fahrenheit (800 degrees Celsius) found in the ancient mantle.
To test the idea, the scientists carried out a series of high-pressure, high-temperature experiments.
They placed marine sediment samples in a vessel with a rock called peridotite that is the most common kind of rock found in the part of the mantle where diamonds form.
Then they turned up the pressure and the heat, giving the samples time to react with one another in conditions like those found at different places in the mantle.
At pressures between 4 and 6 GPa and temperatures between 1,472 and 2,012 degrees Fahrenheit (800-1,100 degrees Celsius), corresponding to depths of between 74.5 and 112 miles (120-180 km) below the surface, they found salts formed with a balance of sodium and potassium that closely matches the small traces found in diamonds.
“We demonstrated that the processes that lead to diamond growth are driven by the recycling of oceanic sediments in subduction zones,” Dr. Förster said.
“The products of our experiments also resulted in the formation of minerals that are necessary ingredients for the formation of kimberlite magmas, which transport diamonds to the Earth’s surface.”
The team’s results appear in the journal Science Advances.
_____
Michael W. Förster et al. 2019. Melting of sediments in the deep mantle produces saline fluid inclusions in diamonds. Science Advances 5 (5): eaau2620; doi: 10.1126/sciadv.aau2620