A Cedars-Sinai Heart Institute-led research team is planning a clinical trial using cardiosphere-derived cells to regenerate the tissue and improve function of the heart in patients who have suffered heart attacks.
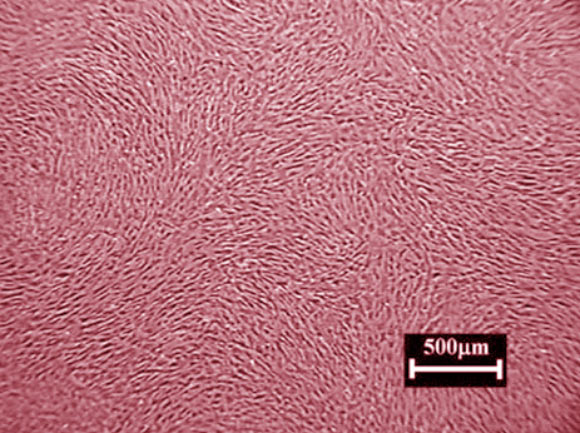
This false-color image shows cardiosphere-derived cells. Image credit: A.J. White et al, doi: 10.1093/eurheartj/ehr172.
Cardiosphere-derived cells (CDCs) are a cluster of proliferating cardiac cells including mesenchymal stem cells.
The study, named the ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) trial, will use allogeneic CDCs – cells derived from donors other than the patients.
In previous pre-clinical and clinical studies, CDCs have been shown to have low immunogenicity and unlikely to result in immune rejection of the cells.
The team’s previous studies have revealed that CDC transplantation was safe and helped improve ventricular function and reduce scar tissue.
“CDC transplantation has been shown to improve the outcome in infarcted myocardium and has an excellent safety profile,” said Dr. Timothy Henry, Director of the Division of Cardiology at Cedars-Sinai Heart Institute and corresponding author of a paper published recently online in the journal Cell Transplantation.
“While autologous transplantation has been shown to curtail immune responses, obtaining CDCs from the patients is not feasible as it is cost-prohibitive and is associated with many logistical complications.”
“Use of other-donated allogeneic cells, however, allows for more efficient processing and transplantation and previous studies have shown significant improvement and tolerability in subjects without the need for immunosuppression.”
The ALLSTAR trial is the first randomized, double-blind, placebo-controlled trial to determine the safety and efficacy of allogeneic CDCs for patients with myocardial infarction (MI).
The study will examine several clinical endpoints that indicate cardiac regeneration, including cardiac and clinical function, quality of life, symptom alleviation, and safety.
Based on the results of the ALLSTAR Phase 1 trial, Dr. Henry and co-authors predict that infusing CDCs will result in improvement for patients with MI.
“Cultural and lifestyle factors, as well as the increasing age of the population, have culminated to make heart health a significant area of interest in medicine,” said Dr. Shinn-Zong (John) Lin, Tzu Chi Hospital, Hualien City, Taiwan, and Co-Editor-in-Chief of Cell Transplantation.
“Cell therapy may play an integral role in the way that heart diseases are treated. This clinical study may hold promise for future approaches for treating MI.”
_____
Tarun Chakravarty et al. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) trial: Rationale & Design. Cell Transplantation, published online August 18, 2016; doi: 10.3727/096368916X692933
This article is based on a press-release from the Cell Transplantation Center of Excellence for Aging and Brain Repair.







