A research team led by MIT scientists has developed an ingestible capsule that can be controlled using Bluetooth, a widely adopted wireless protocol. Manufactured using 3D-printing technology, the capsule could be deployed to deliver drugs to treat a variety of diseases. It could also be designed to sense infections, allergic reactions, or other events, and then release a drug in response.
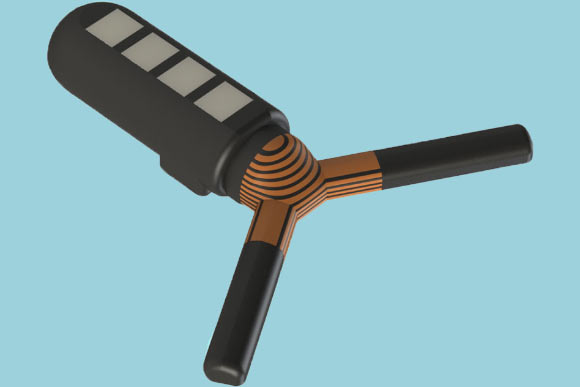
Kong et al designed an ingestible sensor that can lodge in the stomach for a few weeks and communicate wirelessly with an external device. Image credit: Kong et al.
“Our system could provide closed-loop monitoring and treatment, whereby a signal can help guide the delivery of a drug or tuning the dose of a drug,” said study co-lead author Dr. Giovanni Traverso, a visiting scientist in MIT’s Department of Mechanical Engineering.
“We are excited about this demonstration of 3D printing and of how ingestible technologies can help people through novel devices that facilitate mobile health applications,” added MIT Professor Robert Langer, co-lead author of the study.
For the past several years, the team as been working on a variety of ingestible sensors and drug delivery capsules, which they believe would be useful for long-term delivery of drugs that currently have to be injected.
They could also help patients to maintain the strict dosing regimens required for patients with HIV or malaria.
In the latest study, the researchers set out to combine many of the features they had previously developed.
In 2016, they designed a star-shaped capsule with six arms that fold up before being encased in a smooth capsule. After being swallowed, the capsule dissolves and the arms expand, allowing the device to lodge in the stomach.
Similarly, the new device unfolds into a Y-shape after being swallowed. This enables the device to remain the stomach for about a month, before it breaks into smaller pieces and passes through the digestive tract.
One of these arms includes four small compartments that can be loaded with a variety of drugs, which can be packaged within polymers that allow them to be released gradually over several days.
The scientists also anticipate that they could design the compartments to be opened remotely through wireless Bluetooth communication.
The device can also carry sensors that monitor the gastric environment and relay information via a wireless signal.
In previous work, the researchers designed sensors that can detect vital signs such as heart rate and breathing rate.
In the new study, they demonstrated that the capsule could be used to monitor temperature and relay that information directly to a smartphone within arm’s length.
“The limited connection range is a desirable security enhancement,” said study first author Dr. Yong Lin Kong, a researcher at the University of Utah.
“The self-isolation of wireless signal strength within the user’s physical space could shield the device from unwanted connections, providing a physical isolation for additional security and privacy protection.”
To enable the manufacturing of all of these complex elements, the study authors decided to 3D print the capsules.
This approach allowed them to easily incorporate all of the various components carried by the capsules, and to build the capsule from alternating layers of stiff and flexible polymers, which helps it to withstand the acidic environment of the stomach.
“Multimaterials 3D printing is a highly versatile manufacturing technology that can create unique multicomponent architectures and functional devices, which cannot be fabricated with conventional manufacturing techniques,” Dr. Kong said.
“We can potentially create customized ingestible electronics where the gastric residence period can be tailored based on a specific medical application, which could lead to a personalized diagnostic and treatment that is widely accessible.”
The team’s work appears in the journal Advanced Materials Technologies.
_____
Yong Lin Kong et al. 3D-Printed Gastric Resident Electronics. Advanced Materials Technologies, published online December 13, 2018; doi: 10.1002/admt.201800490