A team of scientists at the Schepens Eye Research Institute and Massachusetts Eye and Ear Infirmary has resurrected an ancient adeno-associated virus that is highly effective at delivering gene therapies to the liver, muscle, and retina.
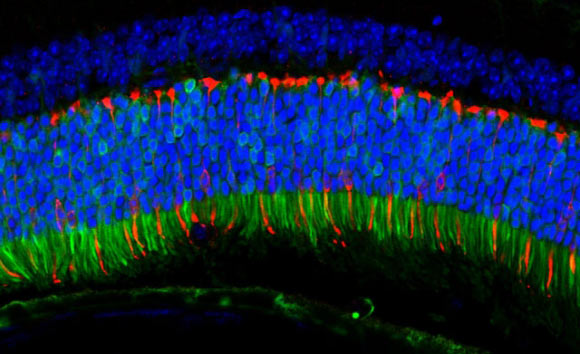
The ANC80 virus delivered genes to the mouse retina that fluoresce green when expressed. Pictured here, the delivered genes are active in the retina’s color-detecting cells. Image credit: Livia Carvalho.
Given its basic nature, a virus can be an ideal delivery system for gene therapy.
In order to survive, a virus must infiltrate a host organism undetected and transfer its genetic material into the host’s cells, where it will use the host to replicate and proliferate.
Taking advantage of this, scientists can insert therapeutic genes into a virus, then use the viruses to shuttle the genes to the appropriate cells or tissues inside a human body.
So far, adeno-associated viruses used for gene therapy have been chosen from viruses that naturally circulate throughout the human population.
If patients have been exposed to the virus, their bodies will likely recognize the virus, mount an attack, and destroy it before it can deliver the therapy.
Engineering new, benign viruses could render the viruses unrecognizable and increase the number of people for whom a given gene therapy will work.
However, efforts to engineer improved adeno-associated viruses have been stymied by the intricate structure of these viruses.
Like pieces of a jigsaw puzzle, every protein in the shell of the virus must fit together perfectly for the virus to function normally. Altering proteins in one part of the virus to achieve a certain benefit, such as more efficient gene transfer or reduced recognition by host immune cells, could end up destroying the structural integrity of the entire shell.
To overcome this challenge, Dr Luk Vandenberghe and co-authors have turned to evolutionary history for guidance.
Over time, ancestors of adeno-associated viruses have undergone a series of changes that kept the structural integrity of the virus while slightly altering some of its functions.
The team was able to recreate an evolutionary timeline of the changes and build in the laboratory nine synthetic viruses.
When injected into mice, the most ancient, ANC80, successfully targeted the liver, muscle and retina without producing toxic side effects.
“We believe our findings will teach us how complex biological structures, such as adeno-associated viruses, are built. From this knowledge, we hope to design next-generation viruses for use as vectors in gene therapy,” said Dr Vandenberghe, senior author of a paper published in the journal Cell Reports.
The team plans to characterize the interplay between ANC80 and host throughout evolution and continue to seek improved vectors for clinical applications.
They will also examine its potential for treating liver diseases and retinal forms of blindness.
“The vectors developed and characterized in this study demonstrate unique and potent biology that justify their consideration for gene therapy applications,” Dr Vandenberghe said.
_____
Eric Zinn et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Reports, published online July 30, 2015; doi: 10.1016/j.celrep.2015.07.019







