An international team of physicists from Germany and Japan has made the most precise measurement yet of the proton’s atomic mass. The result is a factor of three more precise compared to the current literature value, however shifted by about three standard deviations.

Proton is a subatomic particle found in the nucleus of every atom. This artist’s impression shows a proton and a neutron. Image credit: Joanna Griffin / Jefferson Lab / Penn State.
“The proton is the nucleus of the hydrogen atom and one of the basic building blocks of all other atomic nuclei,” the researchers said.
“The proton’s mass is an important parameter in atomic physics — it’s one of the factors that affect how the electrons move around the atomic nucleus. This is reflected in the spectra, i.e., the light colors (wavelengths) that atoms can absorb and emit again.”
“By comparing these wavelengths with theoretical predictions, it is possible to test fundamental physical theories.”
“Further, precise comparisons of the masses of the proton and the antiproton may help in the search for the crucial difference between matter and antimatter.”
To determine the mass of a single proton more accurately, the physicists made the exquisitely precise measurement in an advanced Penning trap system.
“Penning traps are well-proven as suitable ‘scales’ for ions,” they explained. “In such a trap, it is possible to confine, nearly indefinitely, single charged particles such as a proton, for example, by means of electric and magnetic fields.”
“Inside the trap, the trapped particle performs a characteristic periodic motion at a certain oscillation frequency.”
“This frequency can be measured and the mass of the particle calculated from it. In order to reach the targeted high precision, an elaborate measurement technique was required.”
The carbon isotope 12C with a mass of 12 atomic mass units is defined as the mass standard for atoms.
“We directly used it for comparison,” said team member Dr. Sven Sturm, from the Max-Planck-Institut für Kernphysik in Heidelberg, Germany.
“First we stored each one proton and one carbon ion (12C6+) in separate compartments of our Penning trap apparatus, then transported each of the two ions into the central measurement compartment and measured its motion.”
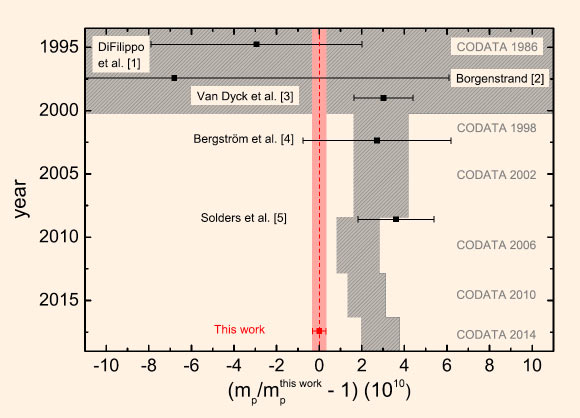
Comparison of the new result to previous values for the proton’s atomic mass. Image credit: Heisse et al, doi: 10.1103/PhysRevLett.119.033001.
From the ratio of the two measured values, the team obtained the mass of proton directly in atomic units.
The measurement compartment was equipped with specifically developed purpose-built electronics.
“It allowed us to measure the proton under identical conditions as the carbon ion despite its about 12-fold lower mass and 6-fold smaller charge,” said team member Dr. Andreas Mooser, of RIKEN’s Fundamental Symmetries Laboratory, Japan.
The resulting mass of the proton — 1.007276466583(15)(29) atomic mass units — is three times more precise than the presently accepted value.
The two numbers given in brackets are the statistical and systematic uncertainties of the measurement, respectively.
“With a precision of 32 parts-per-trillion our result not only improves on the current CODATA (the Committee on Data for Science and Technology) literature value by a factor of three, but also disagrees with it at a level of about 3 standard deviations,” the researchers said.
Measurements by other authors yielded discrepancies with respect to the mass of the tritium atom, the heaviest hydrogen isotope (T), and the mass of light helium (3He) compared to the ‘semiheavy’ hydrogen molecule HD.
“Our result contributes to solving this puzzle, since it corrects the proton’s mass in the proper direction,” said team member Dr. Klaus Blaum, also from the Max-Planck-Institut für Kernphysik.
The research is published in the journal Physical Review Letters (arXiv.org preprint).
_____
F. Heiße et al. 2017. High-Precision Measurement of the Proton’s Atomic Mass. Phys. Rev. Lett 119 (3): 033001; doi: 10.1103/PhysRevLett.119.033001