As many as 33% of autism cases could be explained by a scarcity of a protein called nSR100 in the brain, a new study in the journal Molecular Cell has revealed.
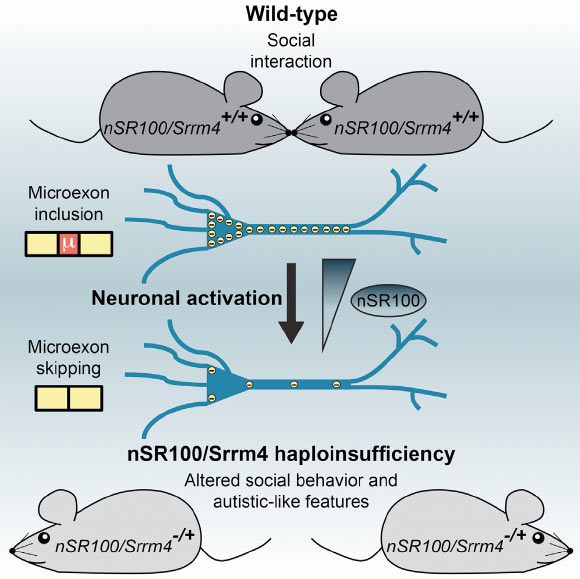
Mathieu Quesnel-Vallières et al induced autistic-like behavior in mice by lowering the levels of nSR100 protein, which is important for normal brain development. Image credit: Mathieu Quesnel-Vallières et al, doi: 10.1016/j.molcel.2016.11.033.
Known best for altered social behaviors, the degree of which can vary tremendously, autism is a common neurological disorder affecting more than 1% of the population.
While its origins are genetic, the specific causes are known in only a fraction of cases that fall into the autism spectrum disorder (ASD). For the majority of people diagnosed with ASD, the reasons behind their disorder remain unknown.
The new study, led by Prof. Benjamin Blencowe of the University of Toronto and Prof. Sabine Cordes of Mount Sinai Hospital and the University of Toronto, provides evidence for the sweeping influence that nSR100 protein, also known as SRRM4, has on social behavior and other features of autism.
In the brain, nSR100 acts as a key regulator of alternative splicing — a process that generates a remarkable diversity of proteins, the building blocks of cells.
“Proteins are encoded in the DNA sequence of the genes, but the useful instructions are broken up and separated by non-coding DNA,” the authors explained.
“During alternative splicing, non-coding spacers are spliced out and protein-coding segments are brought together to make a finished protein template. But the order in which the coding instructions are stitched together can change so that a single gene can spawn a variety of proteins. This way, cells can expand their protein toolbox to vastly outstrip the number of genes.”
“It’s no surprise then, that alternative splicing is especially pronounced in the brain, where the mushrooming protein diversity is thought to be the driving force behind the brain’s astonishing complexity.”
The team previously discovered nSR100 and had shown that it is diminished in the brains of many autistic people.
This finding suggested that autism could, in part, stem from an accumulation of incorrectly spliced proteins in brain cells. This could then lead to mistakes in brain wiring and autistic behavior further down the road.
This time, the researchers decided to test head-on if nSR100 scarcity can indeed cause autism.
“We previously reported an association between nSR100 protein levels and autism,” Prof. Cordes said.
“But this time we show that reduced levels of this protein could really be causative – that’s a big deal. Just by reducing the nSR100 levels by 50%, we observe hallmarks of autistic behavior.”
To do this, the scientists created a mutant mouse that lacks nSR100. They were amazed to find that reducing nSR100 protein levels only by half was enough to trigger the behavioral hallmarks of autism, including avoidance of social interactions and heightened sensitivity to noise.
The nSR100 mutant mice also shared many other features of autism with human patients, such as changes in alternative splicing and brain wiring.
The authors were also able to show that nSR100 levels are linked to neuronal activity.
“If you have an increase in neuronal activity, which is the case in many forms of autism, the nSR100-controlled alternative splicing program is disrupted and this likely underlies autistic behavior,” said study first author Mathieu Quesnel-Vallieres, a graduate student at the University of Toronto.
“A major value of the nSR100 deficient mouse is that it can explain other causes of autism and how they impact neurobiology by converging on the nSR100 protein,” Prof. Blencowe said.
“Our mouse model will also serve as a useful testing ground for small molecules that have potential to reverse nSR100 deficiency in autism.”
“Instead of focusing on individual mutations linked to autism, it’s much more powerful to identify regulatory hubs like nSR100,” Prof. Cordes said.
“In the future, if you turned this protein up a little bit in autistic patients, you might be able to improve some of the behavioral deficits.”
_____
Mathieu Quesnel-Vallières et al. 2016. Misregulation of an Activity-Dependent Splicing Network as a Common Mechanism Underlying Autism Spectrum Disorders. Molecular Cell 64 (6): 1023-1034; doi: 10.1016/j.molcel.2016.11.033







