A team of researchers at the Cincinnati Children’s Heart Institute used an experimental targeted molecular therapy to block a matrix-forming protein, called fibronectin, in heart cells damaged by heart attack, reducing levels of scarred muscle tissue and saving mouse models from heart failure. Their work appears in the journal Circulation.
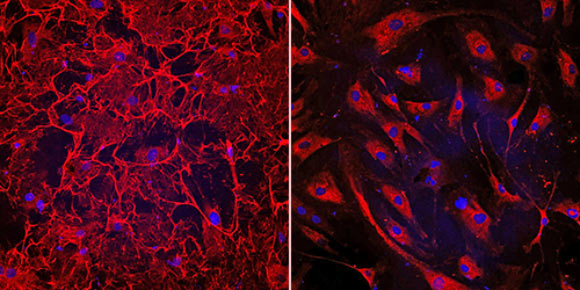
Left: this image shows fibrotic heart cells from a patient who had heart failure; the cells have an elaborate fibronectin matrix (shown in red) which causes fibrosis and heart damage. Right: in this image, human heart cells treated with the novel therapeutic peptide exhibit dramatically reduced fibrosis; the experimental treatment prevented the human heart cells from failing and restored their function. Image credit: Cincinnati Children’s Heart Institute.
The team, headed by Dr. Burns Blaxall, tested a manufactured peptide called pUR4 to block fibronectin in heart cells donated by heart failure patients.
The treatment prevented the human heart cells from failing and restored their function. The treatment also reduced fibrosis and improved heart function after a simulated heart attack in mice.
“Fibronectin is normally a good actor in the body. It helps form a cell-supporting matrix for the body’s connective tissues, aiding tissue repair after injury,” the researchers explained.
“But after a heart attack, this protein overreacts, it polymerizes and helps produce too much connective matrix. It also causes hyperactive production of clogged and dysfunctional cardio myofibroblast cells that damage the heart.”
The pUR4 compound is designed so it will attach to surface points on fibronectin, effectively inhibiting its effects in injured heart cells.
“Our data are a strong proof of principle and the first to show that inhibiting fibronectin polymerization preserves heart function, reduces left ventricle remodeling and limits formation of fibrotic connective tissue,” Dr. Blaxall said.
A key question in the study was verifying the results of pUR4 targeted molecular therapy in both the mouse models and human heart failure cells.
In mice with simulated heart attack that as a control experiment received a placebo therapy, the animals developed significant fibrosis and heart failure.
When the researchers treated mice with pUR4 for just the first seven days after heart attack, or genetically deleted fibronectin activity from the heart cells of mice, these reduced fibrosis and improved cardiac function.
Treatment of human failing heart cells with pUR4 also reduced their fibrotic behavior.
“It’s too early to know whether the experimental therapy in our study can one day be used to treat human heart patients clinically,” the authors said.
“Extensive additional research is needed first, including proving pUR4’s safety in larger animal models and then moving on to establish proof-of-principal effectiveness treating heart failure in those models.”
“We also are working to refine the pUR4 peptide to enhance its capabilities for localized administration to the heart and for extended-release in patients.”
_____
Iñigo Valiente-Alandi et al. Inhibiting Fibronectin Attenuates Fibrosis and Improves Cardiac Function in a Model of Heart Failure. Circulation, published online April 13, 2018; doi: 10.1161/CIRCULATIONAHA.118.034609







