A little known yet powerful function of overactive white blood cells known as neutrophils — the ability to form neutrophil extracellular traps (NETs) — may contribute to organ damage and mortality in COVID-19, according to a study from the NETwork Consortium.
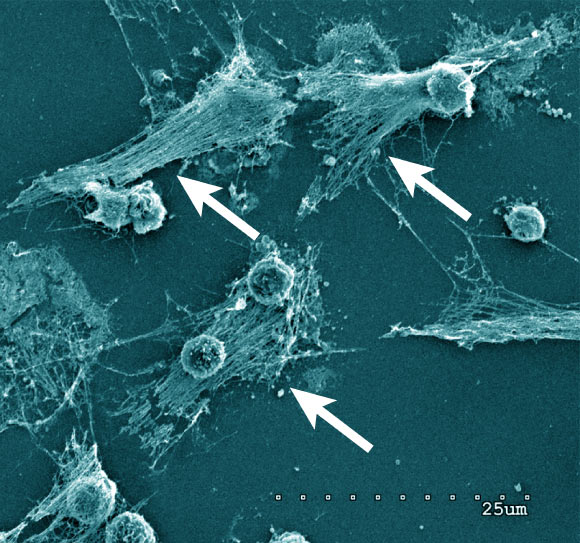
Neutrophils forming NETs in cell culture. Note the expelled DNA strings (arrows). Scanning electron microscopy of neutrophils 3 h after plating and coculturing with 4T1 breast cancer cells. Scale bar – 25 µm. Image credit: Barnes et al, doi: 10.1084/jem.20200652.
Patients with severe COVID-19 infection develop Acute Respiratory Distress Syndrome (ARDS), pulmonary inflammation, thick mucus secretions in the airways, extensive lung damage, and blood clots.
This late stage of the disease is difficult to manage. In the worst cases, patients require invasive mechanical ventilation, and still, a large number of patients die.
This new study suggests that the severity of COVID-19 may result from neutrophils.
Part of the body’s immune system, neutrophils detect bacteria and can expel their DNA to attack the bacteria with a gauzy web of DNA laced with toxic enzymes, called a NET.
These NETs can ensnare and digest the unwanted pathogen but in cases of ARDS, they damage the lungs and other organs.
“Given the clear similarities between the clinical presentation of severe COVID-19 and other known diseases driven by NETs, such as ARDS, we propose that excess NETs may play a major role in the disease,” said Feinstein Institutes Professor Betsy Barnes, lead and co-corresponding author of the study.
“As samples from patients become available, it will be important to determine whether the presence of NETs associates with disease severity and/or particular clinical characteristics of COVID-19.”
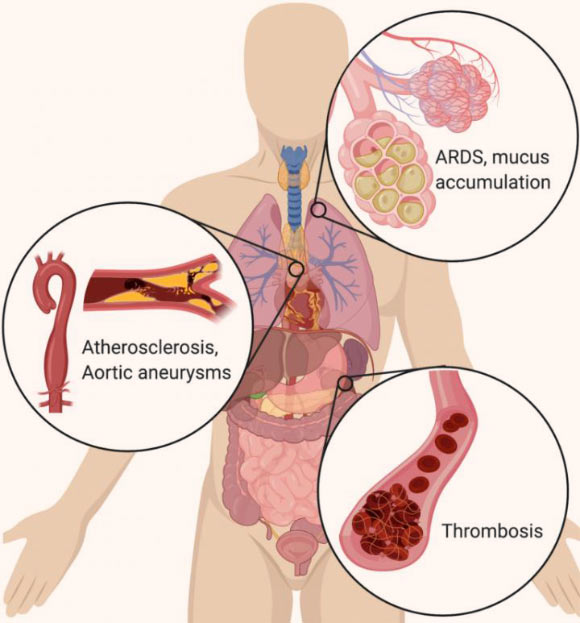
In the lungs, NETs drive the accumulation of mucus in cystic fibrosis patients’ airways. NETs also drive acute respiratory distress syndrome (ARDS) after a variety of inducers, including influenza. In the vascular system, NETs drive atherosclerosis and aortic aneurysms, as well as thrombosis (particularly microthrombosis), with devastating effects on organ function. Image credit: Cold Spring Harbor Laboratory.
“NETs were identified in 2004, but many scientists have never heard of them. Most of the researchers in the NETwork have worked on NETs in other diseases, and when we started hearing about the symptoms of the COVID-19 patients, it sounded familiar,” said Cold Spring Harbor Laboratory cancer biologist Dr. Mikala Egeblad, senior and co-corresponding author of the study.
“We see in these patients severe lung damage known as ARDS, another serious problem caused by excess NETs and seen in cases of severe influenza,” said co-author Dr. Jonathan Spicer, a thoracic surgeon in the Research Institute of the McGill University Health Centre and McGill University.
“In addition, their airways are often clogged with thick mucus and unlike most severe lung infections, these patients tend to form small clots throughout their body at much higher rates than normal.”
“NETs have also been found in the blood of patients with sepsis or cancer, where they can facilitate the formation of such blood clots.”
The NETwork Consortium is now pursuing studies into whether NETs are a common feature in COVID-19 cases.
If the findings show that excess NETs cause the severe symptoms of COVID-19, then a new avenue of treatments may be deployed to help COVID-19 patients.
Current treatments used in other NET and neutrophil-driven diseases — like cystic fibrosis, gout, and rheumatoid arthritis — might dampen the activity of NETs in COVID-19 patients, reducing the need for invasive mechanical ventilation.
The team’s paper was published in the Journal of Experimental Medicine.
_____
Betsy J. Barnes et al. 2020. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 217 (6): e20200652; doi: 10.1084/jem.20200652
This article is based on text provided by Cold Spring Harbor Laboratory.







