A research team at Okayama University in Japan has theoretically predicted a new class of ice phases, called aeroices, likely the most stable solid phases of water near the absolute zero temperature under negative pressure.
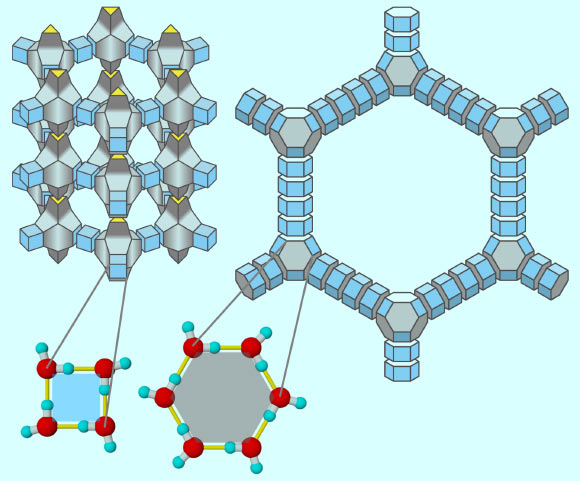
Zeolitic ice (left) and aeroice (right) are illustrated: their structure can be regarded as combinations of a couple of polyhedral building blocks. In molecular scale, each polygonal face of the polyhedra is made of water molecules (left bottom); yellow lines are hydrogen bonds. Image credit: Masakazu Matsumoto / CC BY 2.0.
Water has many ice phases that form under different pressure and temperature conditions. The effects of positive pressure have been explored extensively, with the results somewhat predictable: as the pressure increases, so does the density of the ice.
Much less is known, however, about the effects of extreme negative pressure on water molecules.
Exploring a significant region of negative pressure through molecular dynamic simulations, Okayama University researcher Masakazu Matsumoto and co-authors have now theoretically predicted a new class of ice phases — aeroices.
“Our research, which surveys an entire negative-pressure region for the first time, provides a significant stepping stone in exploring this vast and intricate territory on the phase diagram,” Dr. Matsumoto said.
“Ices with lower density than normal ice are also found to be manifold of many kinds.”
Seventeen ice phases have been found experimentally, each one numbered in the order of its discovery.
In 2014, Dr. Werner Kuhs from the University of Göttingen and co-authors discovered an ice phase that forms under negative pressure: ice XVI.
The molecules of the ice form a zeolite structure, a 3D crystalline cage, in which guest molecules or atoms are trapped inside. The guest molecules (neon particles in this case) were removed, resulting in a stable, ultralow density ice at high negative pressures.
Using a similar technique, a team led by Xiao Cheng Zeng from the University of Nebraska-Lincoln and Jijun Zhao from the Dalian University of Technology discovered ice XVII in 2016.
Dr. Matsumoto and colleagues mapped out all the possible ice phases that might still be left to explore in the negative pressure region.
Knowing that the structure of silica (SiO2) and ice are common, the researchers retrieved 200 silica zeolites from the Zeolite Database.
They rearranged the atoms in the SiO2 structure, removing the two oxygen atoms and replacing the silicon atom in each molecule with one oxygen atom. Then, the hydrogen atoms were added so that the structure obeyed the ice rule.
In the density range that is only around half that of liquid water (0.5 g/cm3), the team showed that the newly discovered ice phase is more stable than any zeolite ice investigated so far.
The authors also simulated even less dense ice structures (0-0.5 g/cm3) by adding polyhedral building blocks to the zeolitic frameworks to make the structure sparser while satisfying the structural rule for ice.
The research is published in the Journal of Chemical Physics.
_____
Takahiro Matsui et al. 2017. Hypothetical ultralow-density ice polymorphs. Journal of Chemical Physics 147 (9); doi: 10.1063/1.4994757







