A team of scientists at North Carolina State University has demonstrated that a gallium-based liquid metal alloy forms snowflake-like fractal patterns when electrochemically oxidized. The results appear in the journal Physical Review Letters.

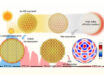
Gallium indium forms fractal patterns with the application of low voltage. Image credit: North Carolina State University.
The team, led by North Carolina State University professors Karen Daniels and Michael Dickey, discovered that applying low voltage to the surface of gallium indium (EGaIn) — a liquid metal with one of the highest surface tensions — causes the liquid metal to spread out and form fractal patterns.
“Applying voltage to EGaIn forms a thin layer of oxide on the surface of the metal, which effectively lowers the surface tension,” Professor Dickey explained.
“Normally, the tension of liquids can be lowered by adding surfactants — like putting soap or detergent in water — to the liquid. It’s easy to put soap into water, but hard to get the soap out. In contrast, the use of voltage to control the tension is interesting because it is reversible, and incredibly effective.”
“We also found that if you apply higher amounts of voltage to the metal it stops spreading and beads up again,” Professor Daniels added.
“That’s due to the amount of oxide produced — a small amount lowers the surface tension, but too much forms a crust over the metal and stops it spreading. So controlling the voltage is a nice way to control the spreading of the metal.”
The team recorded the behavior of EGaIn as the surface tension lowered. Less than one volt of electricity caused the metal to spread out and form different fractals.
Interestingly, the fractals formed by the EGaIn appear to be unique; that is, they do not match any currently described fractals.
“Aside from being unusual, the other implication of these fractals is that in order for them to form the surface tension of the liquid metal must be close to zero,” Professor Daniels said.
“This work suggests that not only does the formation of the oxide lower the surface tension of the liquid metal, but that it also creates compressive stresses — the opposite of tension — that help the metal spread out and form fractals,” Professor Dickey said.
“This is interesting because liquids are always under tension, and we now have a tool to apply compressive forces directly to the surface of a liquid. These properties give us greater control over the metal’s behavior.”
_____
Collin B. Eaker et al. 2017. Oxidation-Mediated Fingering in Liquid Metals. Phys. Rev. Lett 119 (17): 174502; doi: 10.1103/PhysRevLett.119.174502