NASA’s Mars Curiosity rover has detected methane in the Martian atmosphere around it and several organic molecules in a rock sample from its landing site, Gale crater.
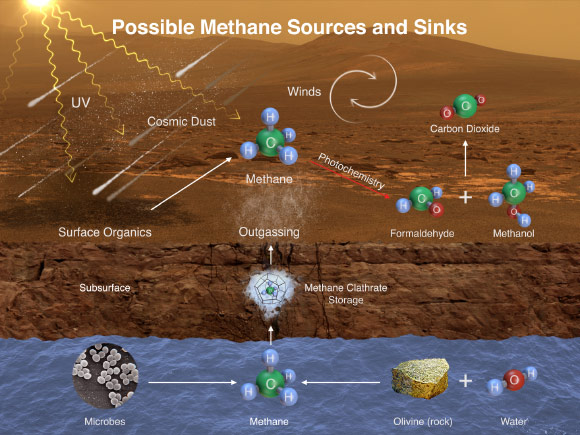
This illustration portrays possible ways that methane might be added to and removed from the Martian atmosphere. Curiosity has detected fluctuations in methane concentration in the atmosphere, implying both types of activity occur in the modern environment of the planet. A molecule of methane consists of one atom of carbon and four atoms of hydrogen. Methane can be generated by microbes and can also be generated by processes that do not require life, such as reactions between water and olivine/pyroxene rock. UV radiation can induce reactions that generate methane from other organic chemicals produced by either biological or non-biological processes, such as comet dust falling on Mars. Methane generated underground in the distant or recent past might be stored within lattice-structured methane hydrates called clathrates, and released by the clathrates at a later time, so that methane being released to the atmosphere today might have formed in the past. Winds on Mars can quickly distribute methane coming from any individual source, reducing localized concentration of methane. Methane can be removed from the atmosphere by sunlight-induced reactions. These reactions can oxidize the methane, through intermediary chemicals such as formaldehyde and methanol, into carbon dioxide, the predominant ingredient in the Martian atmosphere. Image credit: NASA / JPL-Caltech / SAM-GSFC / University of Michigan.
The Curiosity team used the Tunable Laser Spectrometer of the Sample Analysis at Mars (SAM) instrument suite on the rover dozens times in a two-year period to sniff methane in the Martian atmosphere. During two of those months, in late 2013 and early 2014, four measurements averaged seven parts per billion; before and after that, readings averaged only one-tenth that level.
“This temporary increase in methane tells us there must be some relatively localized source. There are many possible sources, biological or non-biological, such as interaction of water and rock,” said team member Dr Sushil Atreya of the University of Michigan, who is a co-author of a paper published in the journal Science.
The scientists also detected different organic chemicals in a drilled sample (dubbed Cumberland) of the Sheepbed mudstone in Gale crater, the landing site for the rover.
They think the crater was once the site of a lake billions of years ago, and rocks like mudstone formed from sediment in the lake. Moreover, this mudstone was found to contain 20 percent smectite clays. On our planet, such clays are known to provide high surface area and optimal interlayer sites for the concentration and preservation of organic compounds when rapidly deposited under reducing chemical conditions.
Organic molecules, which contain carbon and usually hydrogen, are chemical building blocks of life, although they can exist without the presence of life.
Curiosity’s findings do not reveal whether Mars has ever harbored living microbes, but they do shed light on a chemically active modern Mars and on suitable conditions for life in the past.
“We think life began on Earth around 3.8 billion years ago, and our result shows that places on Mars had the same conditions at that time – liquid water, a warm environment, and organic matter. So if life emerged on Earth in these conditions, why not on Mars as well?” said Dr Caroline Freissinet of NASA’s Goddard Space Flight Center in Greenbelt, who is the lead author of a paper submitted to the Journal of Geophysical Research-Planets.
“This first confirmation of organic carbon in a rock on Mars holds much promise,” said team member Dr Roger Summons of the Massachusetts Institute of Technology.
“Organics are important because they can tell us about the chemical pathways by which they were formed and preserved. In turn, this is informative about Earth-Mars differences and whether or not particular environments represented by Gale Crater sedimentary rocks were more or less favorable for accumulation of organic materials.”
The organic molecules detected by the rover also have chlorine atoms, and include chlorobenzene and several dichloroalkanes, such as dichloroethane, dichloropropane and dichlorobutane.
Chlorobenzene is the most abundant with concentrations between 150 and 300 parts-per-billion. It is not a naturally occurring compound on Earth. It is used in the manufacturing process for pesticides (insecticide DDT), herbicides, adhesives, paints and rubber.
Dichloropropane is used as an industrial solvent to make paint strippers, varnishes and furniture finish removers, and is classified as a carcinogen.
It’s possible that these molecules were present as such in the mudstone.
However, it’s more likely that a different suite of precursor organic molecules was in the mudstone, and that the chlorinated organics formed from reactions inside the SAM instrument as the sample was heated for analysis.
Perchlorates (a chlorine atom bound to four oxygen atoms) are abundant on the surface of Mars.
It’s possible that as the sample was heated, chlorine from perchlorate combined with fragments from precursor organic molecules in the mudstone to produce the chlorinated organic molecules detected by SAM.
The scientists also reported that rover’s taste of Martian water, bound into lakebed minerals in the Cumberland rock more than three billion years ago, indicates the planet lost much of its water before that lakebed formed and continued to lose large amounts after.
They analyzed hydrogen isotopes from water molecules that had been locked inside a rock sample for billions of years and were freed when SAM heated it, yielding information about the history of Martian water. The ratio of a heavier hydrogen isotope, deuterium, to the most common hydrogen isotope can provide a signature for comparison across different stages of a planet’s history.
“It’s really interesting that our measurements from Curiosity of gases extracted from ancient rocks can tell us about loss of water from Mars,” said Dr Paul Mahaffy from NASA’s Goddard Space Flight Center in Greenbelt, who is the lead author of the paper reporting the results in the journal Science.
The ratio of deuterium to hydrogen has changed because the lighter hydrogen escapes from the upper Martian atmosphere much more readily than heavier deuterium.
In order to go back in time and see how the deuterium-to-hydrogen ratio in Martian water changed over time, the scientists can look at the ratio in water in the current atmosphere and water trapped in rocks at different times in the planet’s history.
Martian meteorites found on Earth also provide some information, but this record has gaps. No known Martian meteorites are even close to the same age as the rock studied on Mars, which formed about 3.9 billion to 4.6 billion years ago, according to rover’s measurements.
The ratio that Curiosity found in the Cumberland sample is about one-half the ratio in water vapor in today’s Martian atmosphere, suggesting much of the planet’s water loss occurred since that rock formed.
However, the measured ratio is about three times higher than the ratio in the original water supply of Mars, based on assumption that supply had a ratio similar to that measured in Earth’s oceans.
This suggests much of Mars’ original water was lost before the rock formed.
_____
Christopher R. Webster et al. Mars methane detection and variability at Gale crater. Science, published online December 16, 2014; doi: 10.1126/science.1261713
P. R. Mahaffy et al. The imprint of atmospheric evolution in the D/H of Hesperian clay minerals on Mars. Science, published online December 16, 2014; doi: 10.1126/science.1260291







