Genetic scientists from the University of California, Riverside, and the W.M. Keck Science Department of Claremont McKenna, Pitzer and Scripps Colleges, have managed to create a strain of red-eyed mutant wasps.
The red-eyed wasps were created to prove that CRISPR gene-slicing technology can be used on the tiny jewel wasp Nasonia vitripennis, giving scientists a new way to study some of the wasp’s biology.
“No one knows how that selfish genetic element in some male wasps can somehow kill the female embryos and create only males,” said Dr. Omar Akbari, an assistant professor of entomology at the Institute for Integrative Genome Biology at the University of California, Riverside.
“To understand that, we need to pursue their paternal sex ratio (PSR) chromosomes, perhaps by mutating regions of the PSR chromosome to determine which genes are essential for its functionality,” added Dr. Akbari, who is the lead co-author of a paper describing the research, published this week in the journal Scientific Reports.
Enter a gene editing technology known as CRISPR-Cas9, which allows researchers to change any gene.
“Our end goal is to better understand the biology of wasps and other insects, so they can find a way to control insects that destroy crops or spread diseases like malaria,” the authors said.
“But the first step is figuring out how to use the CRISPR technology in such a small organism, something no one had ever done before, in large part because the work is pretty daunting,” Dr. Akbari noted.
“This is because jewel wasps lay their tiny eggs inside a blowfly pupa, which had to be peeled back to expose the teensy eggs.”
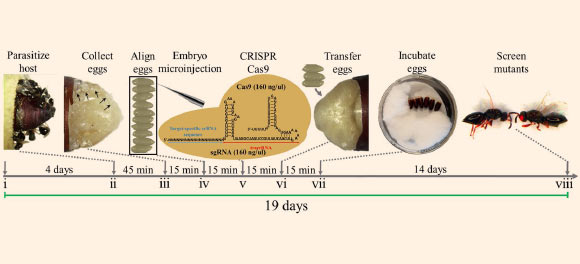
Schematic of Nasonia vitripennis embryo collection and CRISPR/Cas9 microinjections. Adult Nasonia vitripennis were mated for 4 days (i), then were supplied with a flesh fly host pupa, Sarcophaga bullata, for female parasitization for 45 minutes (ii). Embryos were then collected from the host (iii), aligned (iv), and injected with CRISPR/Cas9 components (v). Injected embryos were then gently placed back into the host (vi) for development (14 days) (vii), and when the adults emerged from the host they were subsequently screened for CRISPR/Cas9 induced mutations in target gene (viii). This entire procedure takes roughly 19 days to complete. Image credit: Li et al, doi: 10.1038/s41598-017-00990-3.
In the case of mutant wasps, Dr. Akbari and co-authors decided to slice the eye pigmentation gene cinnabar.
“To establish CRISPR-directed gene editing in Nasonia vitripennis, we targeted a conserved eye pigmentation gene cinnabar, generating several independent heritable germline mutations in this gene,” they explained.
“To generate these mutants, we developed a protocol to efficiently collect Nasonia vitripennis eggs from a parasitized flesh fly pupa, Sarcophaga bullata, inject these eggs with Cas9/guide RNA mixtures, and transfer injected eggs back into the host to continue development.”
The team created a mutant Nasonia vitripennis with heritable traits, which means those red eyes will be passed down to all their offspring in the future – an important quality for researchers who are looking for a stable line of insects to study.
“The technique is challenging, but it is learnable,” Dr. Akbari said. “You need a really steady hand and it requires a lot of patience in micro manipulation that one can learn over time.”
_____
Ming Li et al. 2017. Generation of heritable germline mutations in the jewel wasp Nasonia vitripennis using CRISPR/Cas9. Scientific Reports 7, article number: 901; doi: 10.1038/s41598-017-00990-3