An international team of scientists led by the Rockefeller University has discovered that a variant of the human gene CRY1 (cryptochrome circadian clock 1) slows the internal biological clock, which dictates rhythmic behavior such as sleep/wake cycles. The study was published in the April 6 issue of the journal Cell.
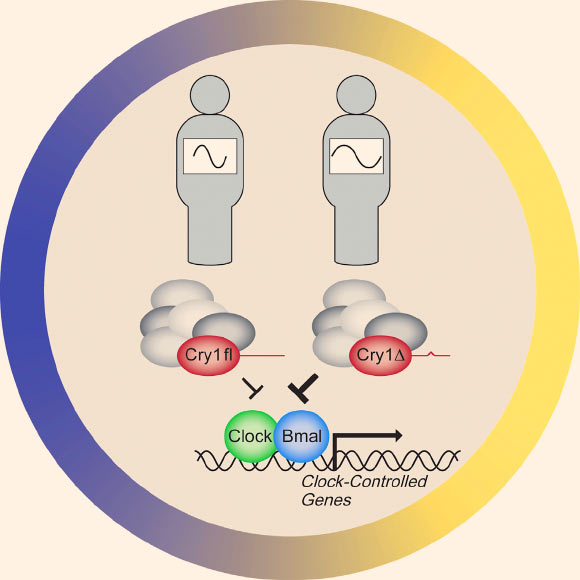
A variation in the human circadian clock gene CRY1 is associated with a familial form of delayed sleep phase disorder, providing genetic underpinnings for ‘night owls.’ Clock and Bmal1 are circadian activator proteins. Image credit: Patke et al, doi: 10.1016/j.cell.2017.03.027.
In the study, carriers of the ‘night owl’ variant experienced nighttime sleep delays of 2-2.5 hours compared to non-carriers.
“Carriers of the mutation have longer days than the planet gives them, so they are essentially playing catch-up for their entire lives,” said lead co-author Dr. Alina Patke, a researcher at the Rockefeller University.
Night owls are often diagnosed at sleep clinics with delayed sleep phase disorder (DSPD).
This study is the first to implicate a gene mutation in the development of DSPD, which affects up to 10% of the public.
People with DSPD often struggle to fall asleep at night, and sometimes sleep comes so late that it fractures into a series of long naps. DSPD and other sleep disorders are associated with anxiety, depression, cardiovascular disease, and diabetes. People with DSPD also have trouble conforming to societal expectations and morning work schedules.
“It’s as if these people have perpetual jet lag, moving eastward every day. In the morning, they’re not ready for the next day to arrive,” said senior author Prof. Michael W. Young, also from the Rockefeller University.
According to the team, not all cases of DSPD are attributable to the ‘night owl’ variant.
However, the authors found it in 1 in 75 of individuals of non-Finnish, European ancestry in a gene database search.
“Normally, the circadian clock begins its cycle by building up proteins, call activators, in a cell,” the researchers explained.
“These activators produce their own inhibitors that, over time, cause the activators to lose their potency.”
“When all the activators in the cell have been silenced, inhibitors are no longer produced and eventually degrade. Once they’ve all gone, the potency of the activators surges, and the cycle begins again.”
“The CRY1 protein is one of the clock’s inhibitors,” they said.
The mutation they found is a single-point mutation in the CRY1 gene, meaning just one letter in its genetic instructions is incorrect. Yet this change causes a chunk of the gene’s resulting protein to be missing.
That alteration causes the inhibitor to be overly active, prolonging the time that the activators are suppressed and stretching the daily cycle by half an hour or more.
In addition to their initial study of a multigenerational family in the United States, the researchers turned to large genetic databases from around the world to determine the prevalence of CRY1 mutations.
With a collaborator in Turkey, they first identified many unrelated families and dozens of Turkish people with the CRY1 mutation.
After contacting them and administering interviews and questionnaires, the scientists were able to confirm that 38 people with the mutation had altered sleep behavior, while none of their relatives without the CRY1 mutation had unusual sleep patterns.
“Right now there’s no established benefit for DSPD patients in being tested for the CRY1 mutation,” the researchers said.
“Just finding the cause doesn’t immediately fix the problem,” Dr. Patke added.
“But it’s not inconceivable that one might develop drugs in the future based on this mechanism,” she said.
_____
Alina Patke et al. 2017. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 169 (2): 203-215; doi: 10.1016/j.cell.2017.03.027