Acne vulgaris, which occurs in 44-95% of teenagers of varying ethnicities, represents a natural, transient inflammatory interaction of adolescent facial skin with new microbes and enhanced production of an oily substance called sebum, according to a new paper published in the journal Trends in Immunology.
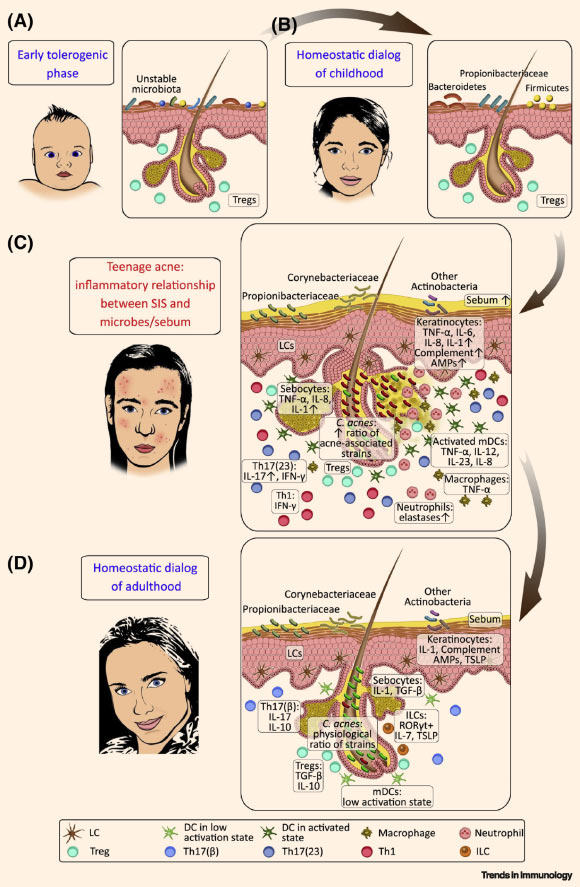
Model of dynamic interactions between the skin immune system (SIS) and its microbial/chemical milieu in human facial skin: (A) infancy — before the development of stabilized microbial communities, an early tolerogenic interaction between the maturing SIS and the microbiota is prevalent; (B) after this initial period, a homeostatic crosstalk evolves between the developed SIS and a childhood-type stable microbiota; (C) adolescence — enhanced sebum secretion and shift in the microbiota composition (and probably other uncovered events, e.g., barrier breach) might contribute to a dynamic change in the communication between the SIS and the microbiota in the skin, leading to inflammatory interactions; cytokines associated with a homeostatic dialog may become highly increased in acne lesions (relative to non-lesions), promoting inflammation together with newly secreted proinflammatory mediators produced by keratinocytes, dendritic cells (DCs), and subsequently, infiltrating inflammatory T helper (Th)1 and Th17(23) (for IL-23) cells, neutrophils, and macrophages; these mediators initiate follicular hyperkeratinization, closure of the follicular infundibulum, comedone formation, and the development of acne papules, pustules, and nodules; the extent and time course of inflammation can vary across a wide spectrum among teenagers; (D) following the resolution of acne, in adult SGR skin, under steady-state, SIS and region-specific microbes carry on a homeostatic dialog with the microbiota again, mediated by the controlled production of innate immune-cell-derived factors, including complement components, AMPs, IL-1, TSLP, and IL-7; hair follicles may play a pivotal role in this crosstalk, supporting the residency and recruitment of LCs, Tregs, ILCs, as well as nonpathogenic Th17(β) cells to maintain homeostasis; upward arrows depict increases (expression, concentrations, and ratios). Propionibacterium acnes (Propionibacteriaceae) = Cutibacterium acnes. Other relevant bacterial genera are noted in the schematic. Abbreviations: AMP – antimicrobial peptide, IL – interleukin, ILC – innate lymphoid cell, LC – Langerhans cell, mDC – myeloid dendritic cell, RORγt – RAR-related orphan receptor γt, SGR – sebaceous gland rich, SIS – skin immune system, TGF – transforming growth factor, Th – T helper, Treg – regulatory T cell, TSLP – thymic stromal lymphopoietin. Image credit: Szegedi et al, doi: 10.1016/j.it.2019.08.006.
Among various inflammatory skin diseases, acne vulgaris is unique because of its specific localization on skin regions rich in sebum-producing sebaceous glands, its occurrence within a narrow age range associated with puberty, its high prevalence in teenagers, and its frequent resolution.
In their paper, Dr. Andrea Szegedi from the University of Debrecen and colleagues propose a new concept that might explain why acne is characterized by strong regional and age specificity, prevalent occurrence, and resolution.
Based on immunological and dermatological data, they hypothesize that the sudden changes in the composition of the microbiota composition within sebaceous-gland-rich skin during adolescence, accompanied by increased sebum production, may result in an inflammatory response that replaces the previous homeostatic host-microbiota crosstalk, thus leading to acne manifestation.
“Our hypothesis, that acne is a naturally developing, transient inflammation state, rather than a pathological skin disease, challenges conventional thinking. This hypothesis incorporates recent scientific data and may explain special clinical characteristics of acne,” Dr. Szegedi said.
The researchers cite evidence in mice showing that even a short-term encounter with new commensal microbes on the skin can initiate the robust accumulation of T lymphocyte white blood cells producing pro-inflammatory cytokines, including interleukin 17 (IL-17) and interferon gamma.
They also highlight messenger RNA data showing that acne lesions contain more pro-inflammatory cytokines characteristic of host-microbiota interactions than healthy skin.
Moreover, acne-associated bacteria can induce both homeostatic and inflammatory states. For example, strains of Cutibacterium acnes associated with acne are capable of activating T cells that produce IL-17 and interferon gamma, whereas other Cutibacterium acnes strains associated with healthy skin promote protective immune responses.
“High sebum production in teenagers seems to be essential for the otherwise commensal Cutibacterium acnes community to initiate inflammation,” the scientists said.
“For example, human macrophages treated with different sebum components secrete significantly elevated concentrations of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor alpha in the presence of Cutibacterium acnes.”
“Consistent with our hypothesis, genome-wide association data in adolescents with severe acne suggest that polymorphisms in inflammatory genes, and genes playing a role in the initiation of tolerance, are associated with disease manifestation,” they added.
“However, one limitation of our framework is that it only applies to acne in adolescence, not in childhood or adulthood.”
_____
Andrea Szegedi et al. Acne: Transient Arrest in the Homeostatic Host-Microbiota Dialog? Trends in Immunology, published online September 26, 2019; doi: 10.1016/j.it.2019.08.006







