An international team of researchers has successfully synthesized a thermodynamically stable compound of sodium and the noble gas helium, Na2He, which has a fluorite-type structure and is stable at high pressures. Their results were published this week in the journal Nature Chemistry.
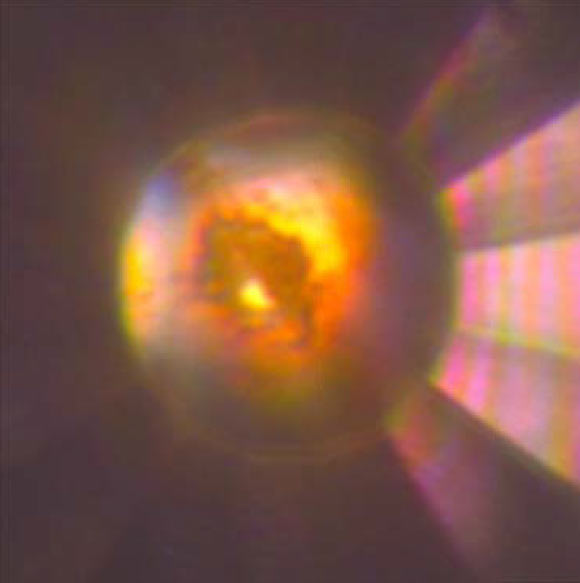
Microphotograph of the diamond anvil cell high-pressure cavity in reflected and transmitted light at 140 GPa after laser heating to > 1,500 degrees Kelvin. The sample, which consists of mixture of tI19 Na and Na2He, is in contact with He medium that is seen as a transparent area near the edge of the gasket. Image credit: Xiao Dong et al, doi: 10.1038/nchem.2716.
“Helium is the second, after hydrogen, most abundant element in the Universe and it is present in significant quantities in normal stars and in gas giant planets such as Jupiter and Saturn,” the researchers said.
“Helium and neon are the most inert elements in the periodic table. This is easy to understand as the ionization potential of the He atom is the highest of all the elements and its electron affinity is zero.”
“The first of the noble gases, helium features an extremely stable, closed-shell electronic configuration, leaving no openings for connections,” added Utah State University Professor Alex Boldyrev, co-author of the study.
Prof. Boldyrev and his colleagues performed a large-scale computational search for possible stable compounds of helium with a variety of elements (H, O, F, Na, K, Mg, Li, Rb, Cs and so on).
They found that only sodium readily forms a stable compound with helium at pressures accessible to static experiments.
“We found a new compound, Na2He, which has lower enthalpy than the mixture of elemental Na and He, or any other mixture, at pressures above 160 GPa (gigapascal),” the authors said.
“The reaction is predicted to be exothermic at pressures above 160 GPa, with reaction enthalpy as large as minus 0.51 eV at 500 GPa.”
“Phonon calculations clearly indicate dynamical stability of Na2He above 100 GPa. This means that, once formed, this phase can be quenched down to 100 GPa, but at and below 50 GPa it is dynamically unstable and therefore unquenchable to ambient conditions.”
The team then experimentally confirmed that sodium readily bonds with helium under high pressure to form the curious Na2He compound.
“The findings were so unexpected that we struggled for more than two years to convince science reviewers and editors to publish our results,” Prof. Boldyrev said.
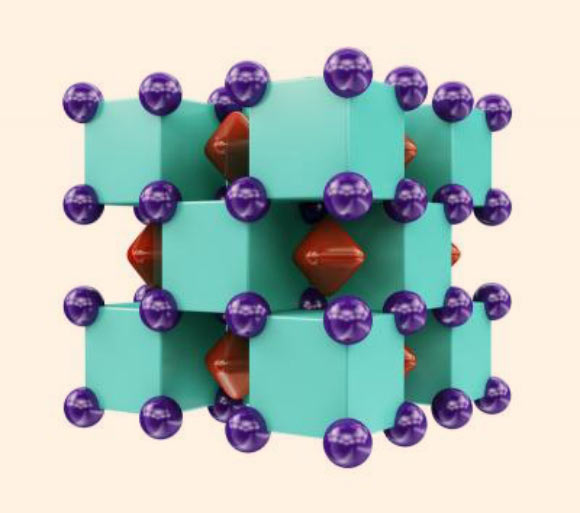
Crystal structure of Na2He: purple spheres represent sodium atoms, which are inside the green cubes that represent helium atoms; the red regions inside voids of the structure show areas where localized electron pairs reside. Image credit: Xiao Dong et al, doi: 10.1038/nchem.2716.
Initially, the Na2He compound was found to consist of Na8 cubes, of which half were occupied by helium atoms and half were empty.
“Yet, when we performed chemical bonding analysis of these structures, we found each ‘empty’ cube actually contained an eight-center, two-electron bond,” Prof. Boldyrev said.
“This bond is what’s responsible for the stability of this enchanting compound.”
The researchers also predict the existence of Na2HeO with a similar structure at pressures above 15 GPa.
_____
Xiao Dong et al. A stable compound of helium and sodium at high pressure. Nature Chemistry, published online February 6, 2017; doi: 10.1038/nchem.2716







