A team of chemists from the Tokyo Institute of Technology and the Okayama University of Science, Japan, has synthesized a ‘nano-Saturn,’ a molecular complex that consists of a spherical C(60) fullerene (the ‘planet’) and a flat macrocycle made of six anthracene units (the ‘rings’). Their work is published in the journal Angewandte Chemie.
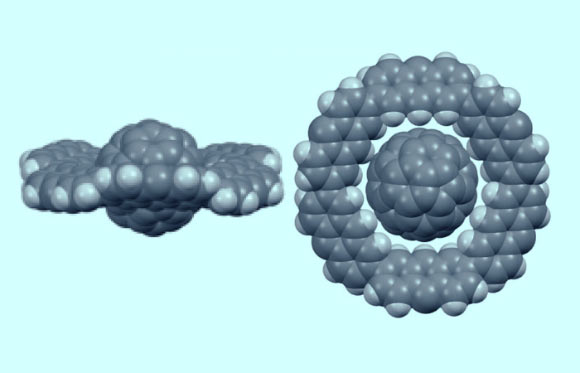
A C(60) molecule is included in a disk-shaped anthracene macrocycle. The formation of a Saturn-type complex is facilitated by the shape-size fit and the multipoint CH***pi-contacts. Image credit: Yamamoto et al, doi: 10.1002/anie.201804430.
“Fullerenes are ideal candidates for the nano-sphere — they are made of carbon atoms linked into a network of rings that form a hollow sphere,” said senior author Professor Shinji Toyota and colleagues.
“The rings must have a rigid, circular form, and must hold the molecular sphere firmly in its midst.”
“The most famous fullerene, buckminsterfullerene C(60), consists of 60 carbon atoms arranged into 5- and 6-membered rings like the leather patches of a classic soccer ball,” the researchers added.
“The electrons in their double bonds, knows as the pi-electrons, are in a kind of electron cloud, able to freely move about and have binding interactions with other molecules, such as a macrocycle that also has a cloud of pi-electrons.”
“The attractive interactions between the electron clouds allow fullerenes to lodge in the cavities of such macrocycles.”
Because of the positions of the electron clouds around the macrocycles, it was previously only possible to make rings that surround the fullerene like a belt or a tire. The rings around Saturn, however, are not like a belt or tire, they are a very flat disc.
“We wanted to properly imitate this at nanoscale. Our success resulted from a different type of bonding between the ‘nano-planet’ and its ‘nano-rings’,” Professor Toyota and co-authors said.
“Instead of using the attraction between the pi-electron clouds of the fullerene and macrocycle, we used the weak attractive interactions between the pi-electron cloud of the fullerene and non-pi-electron of the carbon-hydrogen groups of the macrocycle.”
“To construct the Saturn rings, we chose to use anthracene units, molecules made of three aromatic six-membered carbon rings linked along their edges.”
“We linked six of these units into a macrocycle whose cavity was the perfect size and shape for a C(60) fullerene.”
“Eighteen hydrogen atoms of the macrocycle project into the middle of the cavity. In total, their interactions with the fullerene are enough to give the complex enough stability, as shown by computer simulations.”
By using X-ray analysis and NMR spectroscopy, the scientists was able to prove experimentally that they had produced molecular ‘nano-Saturns.’
_____
Yuta Yamamoto et al. Nano-Saturn: Experimental Evidence of Complex Formation of an Anthracene Cyclic Ring with C60. Angewandte Chemie, published online May 30, 2018; doi: 10.1002/anie.201804430



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)



