A large multinational team of researchers has documented very high levels of molecular chlorine – as high as 400 parts per trillion – in the atmosphere above Barrow, Alaska.
Their study, reported in the journal Nature Geoscience, is the first time that molecular chlorine has been measured in the Arctic, and the first time that scientists have documented such high levels of molecular chlorine in the atmosphere.
The team directly measured molecular chlorine levels in the Arctic in the spring of 2009 over a 6-week period using chemical ionization mass spectrometry.
The level of molecular chlorine above Barrow was measured up to 400 pptv, which is a high concentration considering that chlorine atoms are short-lived in the atmosphere because they are strong oxidants and are highly reactive with other atmospheric chemicals.
Molecular chlorine concentrations (from sea salt released by melting sea ice) peaked in the early morning and late afternoon, and fell to near-zero levels at night.
Average daytime molecular chlorine levels were correlated with ozone concentrations, suggesting that sunlight and ozone may be required for molecular chlorine formation.
Previous studies have documented high levels of oxidized mercury in Barrow – the largest city of the North Slope Borough in the US state of Alaska – and other polar regions. The major source of elemental mercury in the Arctic regions is coal-burning plants around the world.
In the spring in Barrow, ozone and elemental mercury are often depleted from the atmosphere when halogens – chlorine and bromine – are released into the air from melting sea ice.
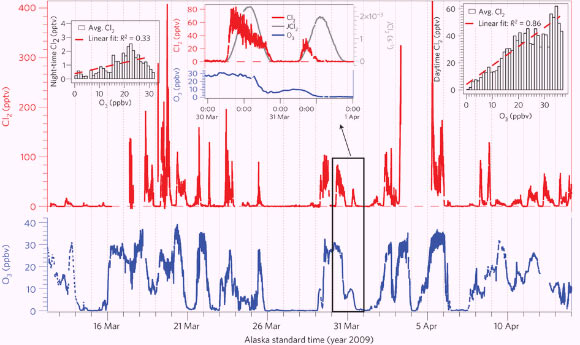
This chart shows the levels of atmospheric chlorine and ozone as measured from Barrow, Alaska, in the spring of 2009. The vertical grid indicates midnight in Alaska Standard Time. Middle inset: chlorine and ozone mixing ratios and chlorine photolysis rate on 30 and 31 March 2009. Right inset: the correlation between average daytime chlorine and ozone. Left inset: the correlation between average night-time chlorine and ozone.
“Molecular chlorine is so reactive that it’s going to have a very strong influence on atmospheric chemistry,” said study second author Prof Greg Huey of the Georgia Institute of Technology in Atlanta.
Chlorine atoms are the dominant oxidant in Barrow. The area is part of a region with otherwise low levels of oxidants in the atmosphere, due to the lack of water vapor and ozone, which are the major precursors to making oxidants in many urban areas.
In Barrow, snow-covered ice pack extends in every directly except inland. The ultimate source of the molecular chlorine is the sodium chloride in sea salt, Huey said, most likely from the snow-covered ice pack. How the sea salt is transformed into molecular chlorine is unknown.
Scientists do know that sea ice is rapidly changing. The sea ice that lasts from one winter to the next winter is decreasing. This has created a larger area of melted ice, and more ice that comes and goes with the seasons. This seasonal variation in ice could release more molecular chlorine into the atmosphere.
______
Jin Liao et al. High levels of molecular chlorine in the Arctic atmosphere. Nature Geoscience, published online January 12, 2014; doi: 10.1038/ngeo2046








![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)