Post-reproductive Caenorhabditis elegans nematode mothers exhibit a form of primitive lactation, releasing yolk milk through their vulva that can enhance growth and reproduction of their larvae, according to new research by University College London scientists.
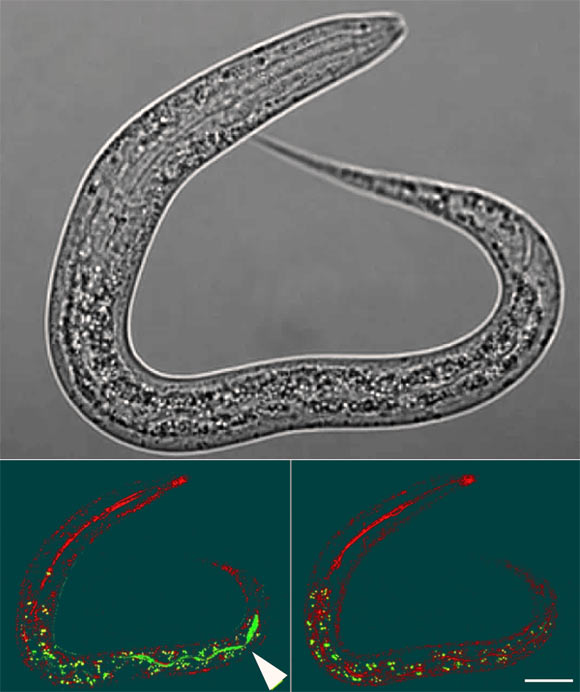
Yolk in the intestinal lumen of Caenorhabditis elegans larva after being left on a plate with no food apart from vented yolk for 4 h. Top: Nomarski microscopy image. Bottom left: larva imaged immediately after removal from plates using reflectance confocal microscopy (RCM) to highlight the refractive material of the terminal web that surrounds the intestinal lumen (red) and superimposed airyscan image (green) for GFP-labeled yolk. Bottom right: same larva imaged 100 min later showing yolk no longer in the lumen, either due to defecation or digestion; this disappearance confirms that the green fluorescence indicated in the central panel corresponds to yolk within the intestinal lumen. Scale bar – 20 μm. Image credit: Kern et al., doi: 10.1038/s41467-021-25821-y.
“We have now explained a unique self-destructive process seen in nematode worms,” said Professor David Gems, a researcher in the Institute of Healthy Ageing at University College London.
“It is both a form of primitive lactation, which only a few other invertebrates have been shown to do, and a form of reproductive suicide, as worm mothers sacrifice themselves to support the next generation.”
Most Caenorhabditis elegans nematodes have both male and female reproductive organs, so the mothers reproduce by fertilizing themselves with limited stocks of self-sperm. When these run out, within days of sexual maturity, reproduction ceases.
The nematodes then behave in a way that has puzzled scientists for some time: they generate large quantities of yolk-rich fluid which accumulates in large pools inside their bodies, destructively consuming internal organs in the process. They also lay more than their own body weight in unfertilized eggs.
Scientists previously assumed these changes were futile and represented some form of old-age disease state.
“Once we realized that the post-reproductive worms were making milk, a lot of things suddenly made sense,” said Dr. Carina Kern, also from the Institute of Healthy Ageing at University College London.
“The worms are destroying themselves in the process of transferring nutrients to their offspring.”
“And all those unfertilized eggs are full of milk, so they are acting like milk bottles to help with milk transport to feed baby worms.”
Previously, the researchers showed that late-life yolk production is a self-destructive process.
In long-lived mutant worms, that are specially bred and intensively studied to try to understand aging, self-destructive late-life yolk production and oocyte (unfertilized egg) laying is switched off.
The new study provides a potential explanation for how genes control Caenorhabditis elegans lifespan: by regulating this self-destructive process.
The authors found the milk-like fluid appears to benefit larvae, as they found evidence that larvae were indeed ingesting the worm milk, and larvae that had access to a source of milk — which they also called ‘yolk milk’ — grew more quickly.
“The existence of worm milk reveals a new way that Caenorhabditis elegans maximize their evolutionary fitness: when they can’t reproduce anymore because they have run out of sperm, they melt down their own tissues in order to transfer resources to their offspring,” Dr. Kern said.
The new findings could also have far-reaching implications in terms of the prospects of being able to slow the human aging process.
Self-destructive and life-shortening reproductive effort of this sort is typical of organisms such as Pacific salmon that exhibit suicidal reproduction. The new study suggests Caenorhabditis elegans lifespan may too be limited by suicidal reproduction.
“The amazing thing about aging in Caenorhabditis elegans is that lifespan can be massively increased by gene manipulation — up to 10-fold,” Professor Gems said.
“This suggests that by understanding how this happens, one could find the key to slowing human aging, which is really exciting.”
“But if Caenorhabditis elegans life extension is just due to suppression of suicidal reproduction like in salmon, then the possibility of applying our knowledge of worm aging to dramatically extend human life suddenly looks remote.”
The study was published in the journal Nature Communications.
_____
C.C. Kern et al. 2021. C. elegans feed yolk to their young in a form of primitive lactation. Nat Commun 12, 5801; doi: 10.1038/s41467-021-25821-y