Alzheimer’s disease is the most well-known and most common type of age-related dementia. Amyloid plaques and tau tangles are both pathological hallmarks of the disease. A new study in mice shows that a novel DNA vaccine delivered to the skin prompts an immune response that reduces buildup of tau and beta-amyloid proteins.
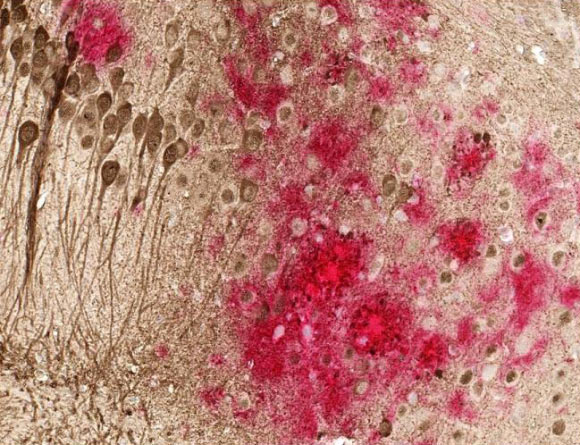
Toxic amyloid plaques (red) and tau tangles (brown) form on the brain of a mouse modeled to have Alzheimer’s disease. Image credit: University of Texas Southwestern.
The new vaccine is on a shortlist of promising antibody treatments aimed at protecting against both types of proteins that kill brain cells as they spread in deadly plaques and tangles on the brains of Alzheimer’s disease patients.
“Our study is the culmination of a decade of research that has repeatedly demonstrated that this vaccine can effectively and safely target in animal models what we think may cause Alzheimer’s disease. I believe we’re getting close to testing this therapy in people,” said study lead author Dr. Roger Rosenberg, founding Director of the Alzheimer’s Disease Center at the University of Texas Southwestern.
Although earlier research established that antibodies significantly reduce amyloid buildup in the brain, Dr. Rosenberg and colleagues needed to find a safe way to introduce them into the body.
A vaccine showed promise in the early 2000s, but when tested in humans, it caused brain swelling in some patients.
The team’s idea was to start with DNA coding for amyloid and inject it into the skin rather than the muscle to produce a different kind of immune response.
The injected skin cells make a three-molecule chain of beta-amyloid (Aβ42), and the body responds by producing antibodies that inhibit the buildup of amyloid and indirectly also of tau.
The study — consisting of four cohorts of between 15 and 24 mice each — shows the vaccine prompted a 40% reduction in beta-amyloid and up to a 50% reduction in tau, with no adverse immune response.
“If amyloid and tau are indeed the cause of Alzheimer’s disease, achieving these reductions in humans could have major therapeutic value,” Dr. Rosenberg said.
“If the onset of the disease could be delayed by even five years, that would be enormous for the patients and their families. The number of dementia cases could drop by half,” said study senior author Dr. Doris Lambracht-Washington, also from the University of Texas Southwestern.
“Allowing the body to produce its own antibodies through active immunization would be the preferable strategy, if it can be done safely. Among the advantages, the vaccine would be more accessible and less expensive,” Dr. Rosenberg added.
“It also produces a wider variety of antibody types than the preformed antibodies containing only one specific antibody.”
The findings are published in the journal Alzheimer’s Research and Therapy.
_____
Roger N. Rosenberg et al. 2018. Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology. Alzheimer’s Research & Therapy 10: 115; doi: 10.1186/s13195-018-0441-4







