Researchers from the University of Oxford and PsiOxus Therapeutics have successfully adapted an oncolytic adenovirus that specifically kills cancer cells, to synthesize a peptide linker that approximates cytotoxic T-cells to cancer-associated fibroblasts in order to eliminate these supportive accomplices.
Cancer cells (black) and nearby fibroblasts (red) being killed (green flashes) by Enadenotucirev equipped with a bispecific T-cell engager. The cancer cells are infected by the virus and actively secrete the bispecific T-cell engager, before dying. The T-cell engager causes the T cells (blue) to attach to the fibroblasts and kill them. Credit: J. Freedman / Cancer Research.
The tumor microenvironment (TME) is made up of cellular and acellular components that provide the physical niche in which the cancer resides, such as stromal and immune cells, blood vessels, signaling factors, and the extracellular matrix.
Prolific research in the field has been instrumental in showing that the TME is crucial for tumorigenesis as well as cancer development.
Fibroblasts are types of stromal cells that modulate the TME by producing pro- or anti-inflammatory signals, which act to recruit or repel lymphocytes, respectively.
Specifically, cancer-associated fibroblasts (CAF) can maintain an immunosuppressive environment by releasing anti-inflammatory chemokines and metabolites, thus coordinating depressed cytotoxic T-cell proliferation and activity.
These finding have furthered interest in targeting the TME in combination with the cancer itself to establish a durable treatment response.
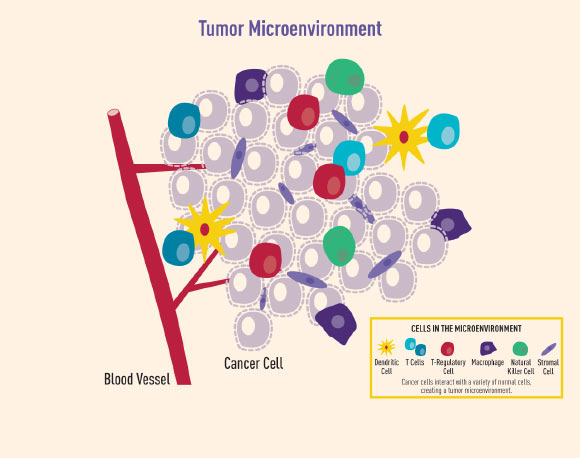
The cellular compartment of the tumor microenvironment is made up of tumor cells, immune cells, and stromal cells. Image credit: National Cancer Institute.
Dr. Joshua Freedman and colleagues developed a two-pronged approach to treatment that utilizes a carcinoma-specific oncolytic group B adenovirus, Enadenotucirev (EnAd). EdAd specifically replicates in and kills carcinomas over normal cells, thereby causing cell death by oncosis, or membrane integrity disruption.
Additionally, an in vitro study demonstrated that EnAd-mediated cell death causes the release of pro-inflammatory and pro-phagocytic signals that activate dendritic cells and stimulate T cells, and a Phase I clinical trial has shown that EnAd has global immune-stimulating properties.
The key innovation of this study was the coupling of these approaches, via the addition of a genomic sequence in the EnAd that codes for a Bi-specific T-cell Engager (BiTE), a peptide that activates cytotoxic T-cells and directs them towards target cells with a specific surface marker.
BiTEs have antibody-like regions which bind antigens of selected target cells on one end, and activate the CD3ε receptor of the T-cell on the other.
This BiTE used the high-density CAF marker FAP, which is associated with immunosuppression and thus a particularly attractive target of immune activation.
BiTEs are FDA-approved in B-ALL, and this research group has previously coupled oncolytic viruses with BiTEs targeting cancerous antigens, but this is the first to target the neighboring stromal cells.
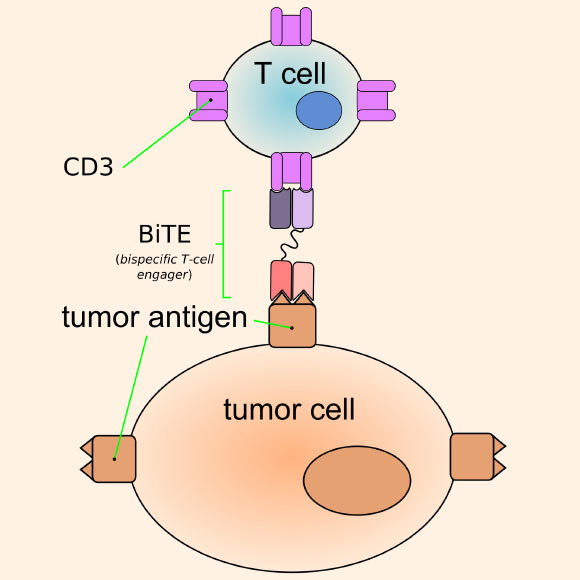
Bi-specific T-cell Engager (BiTE) can bring together a T-cell and a cell of interest, a tumor cell in this example, promoting T-cell killing of the target cell. Image credit: Armin Kübelbeck / CC BY-SA 3.0.
Study leader Dr. Kerry Fisher highlights the important finding of this work, “Until now, there has not been any way to kill both cancer cells and the fibroblasts protecting them at the same time, without harming the rest of the body.”
“This novel modality of therapy can be critical for patients with ‘cold’ tumors, which are effectively hidden from the immune system and suffer from a lack of therapeutic options.”
Dr. Nathan Richardson, the head of Molecular and Cellular Medicine at the Medical Research Council, says, “Immunotherapy is emerging as an exciting new approach to treating cancers. This innovative viral delivery system, which targets both the cancer and surrounding protective tissue, could improve outcomes for patients whose cancers are resistant to current treatments.”
The results are published in the journal Cancer Research.
_____
Joshua D. Freedman et al. An Oncolytic Virus Expressing a T-cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer Research, published online November 18, 2018; doi: 10.1158/0008-5472.CAN-18-1750







