A duo of researchers from Columbia University and Argonne National Laboratory has discovered an entirely new class of chemical reaction, dubbed chemically termolecular (i.e. three-molecule) reactions, in which three different molecules are involved in bond making and/or breaking.
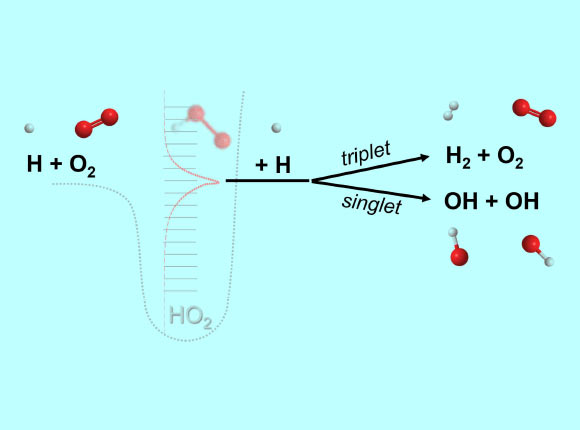
A chemically termolecular reaction is a reaction where three different molecules (e.g. H, O2, H) each participate in the breaking and forming of chemical bonds. The reaction is mediated by an ephemeral collision complex (HO2**) formed from the collision of two molecules (H, O2) which then reacts upon colliding with a third molecule (H). Image credit: Michael P. Burke / Columbia Engineering.
‘Chemically termolecular’ reactions were first hypothesized in the 1920-30s by Sir Cyril Norman Hinshelwood and Nikolay Nikolaevich Semenov in their studies of chain reactions (they won the 1956 Nobel Prize in Chemistry).
For decades, chemists have considered such reactions unimportant — if they even occurred at all — and until now, no one has studied them.
Columbia University researcher Dr. Michael Burke decided to investigate these reactions after realizing that common combustion situations, such as those encountered in many engines, have sufficiently high fractions of highly reactive molecules known as free radicals to make these reactions possible.
“Combustion has always been a launching point for understanding all sorts of other chemical mechanisms,” Dr. Burke explained.
“Potentially there could be innumerable reactions from this new class that impact how we model gas phase chemistry, from designing new types of engines to understanding the planetary chemistry responsible for cloud formations, climate change, evolution of pollutants, even perhaps the sequence of reactions that could impact the conditions for extraterrestrial life. Our discovery opens up a whole new world of possibilities.”
“For example, space vehicles experience very high temperatures and radical fractions in their descent back to Earth. This fourth class of reactions could impact the heat flux to the vehicle, with significant implications for the design of thermal protection systems to keep astronauts and/or payloads safe when coming down to Earth.”
Working with Argonne National Laboratory theoretical chemist Dr. Stephen Klippenstein, Dr. Burke used computational methods, combining quantum chemical computations that simulate the breaking and forming of chemical bonds among reacting molecules with kinetic-transport computations that simulate the reactions and movements of bulk gases that governs performance of engineering devices.
“Our calculations are based on computational data produced from first principles: the Schrödinger equation, the fundamental equation of quantum mechanics,” Dr. Burke said.
“Combining these data with other physics-based models enables us to directly pinpoint the impact of just a single reaction out of many, in a way that is very difficult to do in the lab.”
The team showed that these chemically termolecular reactions not only are major chemical pathways but also impact flame propagation speeds, a measure of overall fuel reactivity that governs the performance, stability, and efficiency of many modern engines.
Up to now, only three classes of reactions have been considered:
(i) unimolecular reactions, where one reactant undergoes bond breaking and/or forming to yield different products;
(ii) bimolecular reactions, where two reactants collide and then undergo bond breaking and/or forming to yield different products;
(iii) termolecular association reactions, where two reactants collide to form a molecular complex with a new chemical bond between the two reactants and a third molecule, known as the bath gas, removes some of the internal kinetic energy of that molecule to stabilize it.
“The bath gas is usually considered an inert, or non-reactive, molecule that does not participate in any bond breaking or forming, but instead takes away some energy from the other molecular complex,” the authors explained.
“If instead the molecular complex collides with a reactive molecule, then the third molecule can participate in the bond-breaking/forming process, yielding what we call a ‘chemically termolecular reaction’ product.”
Dr. Burke added: “we showed the importance of reactions involving H + O2 complexes with other radical species, e.g. H + O2 + H, in combustion environments.”
“However, given the fact that reactive molecules, like free radicals and molecular oxygen, are major constituents in combustion and certain planetary environments, there is significant potential for other chemically termolecular reactions to occur and to play a significant role in other environments.”
The research is published in the journal Nature Chemistry.
_____
Michael P. Burke & Stephen J. Klippenstein. Ephemeral collision complexes mediate chemically termolecular transformations that affect system chemistry. Nature Chemistry, published online August 14, 2017; doi: 10.1038/nchem.2842



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)



