A research team at the University of Connecticut, led by Professor Douglas Adamson, has developed and patented a one-of-a-kind process for exfoliating graphene in its pristine (pure, unoxidized) form, as well as manufacturing innovative graphene nanocomposites.
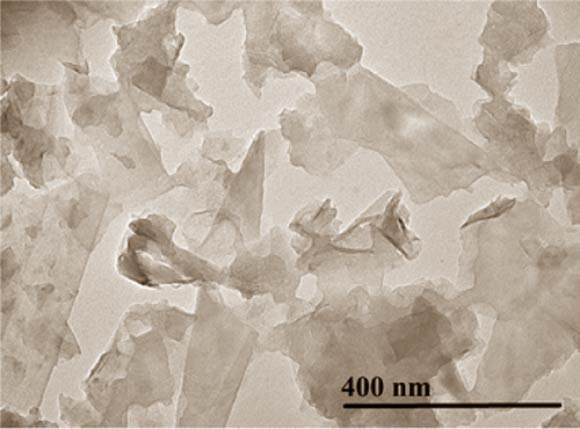
Professor Adamson and co-authors demonstrated the use of an interfacial trapping technique to assemble laterally macroscopic films of pristine graphene that are up to 95% transparent. Image credit: Woltornist et al, doi: 10.1021/nn402371c.
Graphene is comprised of a single layer of densely packed carbon atoms, arranged in a hexagonal lattice.
This material is at least 100 times stronger than steel. Aerogels made from graphene are some of the lightest materials known to man, and the graphene sheets are one of the thinnest, at only one atom thick.
Graphene is also even more thermally and electrically conductive than copper, with minimal electrical charge.
Because of these unique qualities, graphene has been a hot topic for academic researchers and industry leaders since it was first isolated from graphite in 2004.
What others are calling ‘graphene’ is often actually graphene oxide that has been chemically or thermally reduced.
The oxygen in graphene oxide provides a sort of chemical handle that makes the graphene easier to work with, but adding it to pristine graphene reduces the material’s mechanical, thermal, and electrical properties in comparison to unmodified graphene like the kind Professor Adamson and colleagues produce.
It also significantly increases the cost to manufacture the material. Oxidizing graphite requires adding expensive hazardous chemicals, such as anhydrous sulfuric acid and potassium peroxide, followed by a lengthy series of manipulations to isolate and purify the products, known as a chemistry workup.
The team’s process doesn’t require any additional steps or chemicals to produce graphene in its pristine form.
“The innovation and technology behind our material is our ability to use a thermodynamically driven approach to un-stack graphite into its constituent graphene sheets, and then arrange those sheets into a continuous, electrically conductive, three-dimensional structure,” Professor Adamson said.
“The simplicity of our approach is in stark contrast to current techniques used to exfoliate graphite that rely on aggressive oxidation or high-energy mixing or sonication – the application of sound energy to separate particles — for extended periods of time.”
“As straightforward as our process is, no one else had reported it. We proved it works.”
The results of this research were published in the journal ACS Nano.
_____
Steven J. Woltornist et al. 2013. Conductive Thin Films of Pristine Graphene by Solvent Interface Trapping. ACS Nano 7 (8): 7062-7066; doi: 10.1021/nn402371c



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)



