A collaboration of neuroscientists from the United States, China and Europe has used a wireless ‘brain-spine interface’ to bypass spinal cord injuries in rhesus macaques (Macaca mulatta), restoring intentional walking movement to a temporarily paralyzed leg.
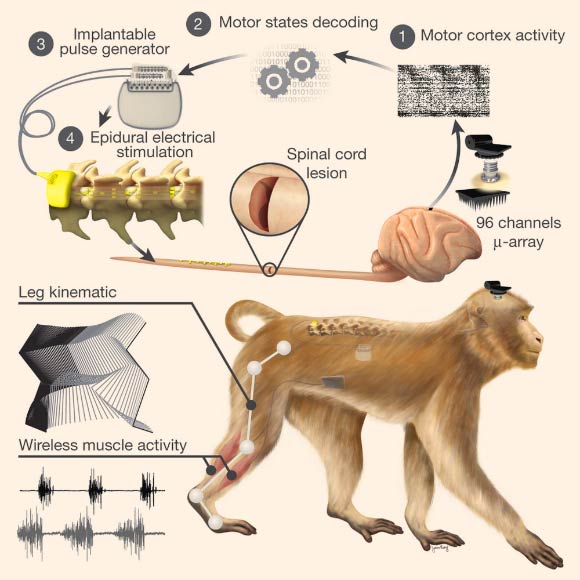
Conceptual and technological design of the brain-spine interface: rhesus macaques (Macaca mulatta) were implanted with a microelectrode array into the leg area of the left motor cortex; during recordings, a wireless module transmitted broadband neural signals to a control computer. (1) a raster plot recorded over three successive gait cycles; each line represents spiking events identified from one electrode, while the horizontal axis indicates time; (2) a decoder running on the control computer identified motor states from these neural signals; (3) these motor states triggered electrical spinal cord stimulation protocols. For this, the monkeys were implanted with a pulse generator featuring real-time triggering capabilities; (4) the stimulator was connected to a spinal implant targeting specific dorsal roots of the lumbar spinal cord. Electromyographic signals of an extensor (grey) and flexor (black) muscles acting at the ankle recorded over three successive gait cycles are shown together with a stick diagram decomposition of leg movements during the stance (grey) and swing (black) phases of gait. Image credit: Jemère Ruby / Marco Capogrosso et al, doi: 10.1038/nature20118.
Walking is made possible by a complex interplay among neurons in the brain and spinal cord. Electrical signals originating in the brain’s motor cortex travel down to the lumbar region in the lower spinal cord, where they activate motor neurons that coordinate the movement of muscles responsible for extending and flexing the leg.
Injury to the upper spine can cut off communication between the brain and lower spinal cord. Both the motor cortex and the spinal neurons may be fully functional, but they are unable to coordinate their activity. The goal of the current study was to re-establish some of that communication.
“The system we have developed uses signals recorded from the motor cortex of the brain to trigger coordinated electrical stimulation of nerves in the spine that are responsible for locomotion,” said co-lead author Prof. David Borton, from Brown University and the Ecole Polytechnique Federale Lausanne (EPFL) in Switzerland.
“With the system turned on, the animals in our study had nearly normal locomotion.”
This is the first time a neural prosthetic has been used to restore walking movement directly to the legs of nonhuman primates.
The results could help in developing a similar system designed for humans who have had spinal cord injuries.
“There are many challenges ahead and it may take several years before all the components of this intervention can be tested in people,” said EPFL researcher Prof. Grégoire Courtine, who led the collaboration.
“There is evidence to suggest that a brain-controlled spinal stimulation system may enhance rehabilitation after a spinal cord injury. This is a step toward further testing that possibility,” Dr. Borton added.

The brain-spine interface developed for this study uses a brain implant like this one to detect spiking activity in the brain’s motor cortex. Seen here, a microelectrode array and a silicon model of a primate’s brain, as well as a pulse generator used to stimulate electrodes implanted on the spinal cord. Image credit: Alain Herzog / EPFL.
The brain-spinal interface developed by the team uses a pill-sized electrode array implanted in the brain to record signals from the motor cortex.
A wireless neurosensor sends the signals gathered by the brain chip wirelessly to a computer that decodes them and sends them wirelessly back to an electrical spinal stimulator implanted in the lumbar spine, below the area of injury. That electrical stimulation, delivered in patterns coordinated by the decoded brain, signals to the spinal nerves that control locomotion.
“Rhesus monkeys were implanted with an intracortical microelectrode array in the leg area of the motor cortex and with a spinal cord stimulation system composed of a spatially selective epidural implant and a pulse generator with real-time triggering capabilities,” the authors said.
To calibrate the decoding of brain signals, they implanted the brain sensor and wireless transmitter in healthy macaques.
The signals relayed by the sensor could then be mapped onto the animals’ leg movements. They showed that the decoder was able to accurately predict the brain states associated with extension and flexion of leg muscles.
“The ability to transmit brain signals wirelessly was critical to this work. Wired brain-sensing systems limit freedom of movement, which in turn limits the information researchers are able to gather about locomotion,” Dr. Borton said.
“Doing this wirelessly enables us to map the neural activity in normal contexts and during natural behavior. If we truly aim for neuroprosthetics that can someday be deployed to help human patients during activities of daily life, such untethered recording technologies will be critical.”
The neuroscientists combined their understanding of how brain signals influence locomotion with spinal maps. That enabled the team to identify the neural circuits that should be stimulated by the spinal implant.
With these pieces in place, they then tested the entire system on two macaques with lesions that spanned half the spinal cord in their thoracic spine.
“Macaques with this type of injury generally regain functional control of the affected leg over a period of a month or so. We tested the system in the weeks following the injury, when there was still no volitional control over the affected leg,” the researchers said.
The study showed that with the system turned on, the animals began spontaneously moving their legs while walking on a treadmill.
Kinematic comparisons with healthy controls showed that the lesioned macaques, with the aid of brain-controlled stimulation, were able to produce nearly normal locomotor patterns.
“While demonstrating that the system works in a nonhuman primate is an important step, much more work must be done to begin testing the system in humans,” the researchers said.
They also pointed out several limitations in the study. For instance, while the system used in this study successfully relayed signals from the brain to the spine, it lacks the ability to return sensory information to the brain.
The authors were also unable to test how much pressure the animals were able to apply to the affected leg. While it was clear that the limb was bearing some weight, it wasn’t clear from this work how much.
“In a full translational study, we would want to do more quantification about how balanced the animal is during walking and measure the forces they’re able to apply,” Dr. Borton said.
The research appears today in the journal Nature.
_____
Marco Capogrosso et al. 2016. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284-288; doi: 10.1038/nature20118
This article is based on a press-release from Brown University.







