In a new study published in the journal Cell Metabolism, researchers took a closer look at the functions of different sensory neurons in the control center of the vagus nerve, which is one of the biggest nerves connecting our gut and brain.
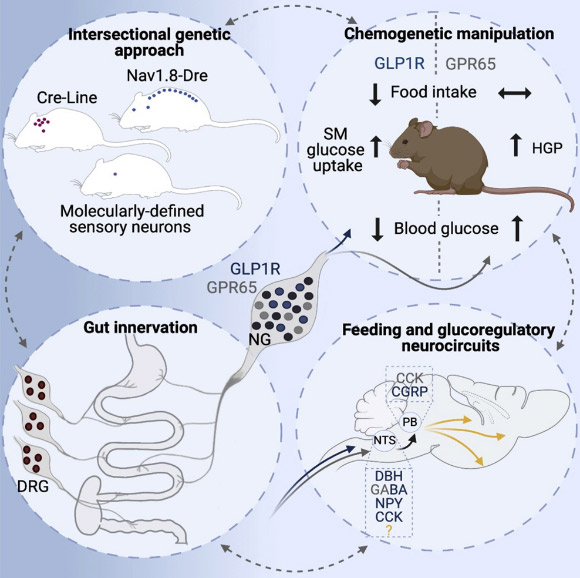
Borgmann et al. employed intersectional genetic manipulations to probe the feeding and glucoregulatory function of distinct sensory neurons; they reconstructed the gut innervation patterns of numerous molecularly defined vagal and spinal afferents and identified their downstream brain targets; bidirectional chemogenetic manipulations, coupled with behavioral and circuit mapping analysis, demonstrated that gut-innervating, glucagon-like peptide 1 receptor (GLP1R)-expressing vagal afferents relay anorexigenic signals to parabrachial nucleus neurons that control meal termination; moreover, GLP1R vagal afferent activation improved glucose tolerance, and their inhibition elevated blood glucose levels independent of food intake; in contrast, gut-innervating, GPR65-expressing vagal afferent stimulation increased hepatic glucose production and activated parabrachial neurons that control normoglycemia, but they were dispensable for feeding regulation. Image credit: Borgmann et al., doi: 10.1016/j.cmet.2021.05.002.
“In the control center of the vagus nerve, the so-called nodose ganglion, various neurons are situated, some of which innervate the stomach while others innervate the intestine,” said study’s senior author Dr. Henning Fenselau, a researcher from the Max Planck Institute for Metabolism Research, the University Hospital Cologne, and the University of Cologne, and his colleagues.
“Some of these neurons detect mechanical stimuli in the different organs, such as stomach stretch during feeding, while others detect chemical signals, such as nutrients from the food that we consume.”
“But what roles these different cells play in transmitting information from the gut to the brain, and how their activity contributes to adaptations of feeding behavior and blood sugar levels had remained largely unclear.”
The scientists developed a genetic approach that enabled them to visualize two types of sensory neurons in the nodose ganglion of mice.
“One of these cell types detects stomach stretch, and activation of these nerve cells causes mice to eat significantly less,” Dr. Fenselau explained.
“We identified that activity of these nerve cells is key for transmitting appetite-inhibiting signals to the brain and also decreasing blood sugar levels.”
“The second group of nerve cells primarily innervates the intestine. It senses chemical signals from our food. However, their activity is not necessary for feeding regulation. Instead, activation of these cells increases our blood sugar level.”
Thus, these two types of nerve cells in the control center of the vagus nerve fulfill very different functions.
“The reaction of our brain during food consumption is probably an interplay of these two nerve cell types,” Dr. Fenselau said.
“Food with a lot of volume stretches our stomach, and activates the nerve cell types innervating this organ.”
“At a certain point, their activation promotes satiety and hence halts further food intake, and at the same time coordinates the adaptations of blood sugar levels.”
“Food with a high nutrient density tends to activate the nerve cells in the intestine.”
“Their activation increases blood glucose levels by coordinating the release of the body’s own glucose, but they do not halt further food intake.”
“The discovery of the different functions of these two types of nerve cells could play a crucial role in developing new therapeutic strategies against obesity and diabetes.”
_____
Diba Borgmann et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metabolism, published online May 26, 2021; doi: 10.1016/j.cmet.2021.05.002







