Dr. Bao-Zhong Wang of the Institute for Biomedical Sciences at Georgia State University and colleagues have developed an experimental nanoparticle vaccine against influenza A virus that seems to work in mice. The findings appear in the journal Proceedings of the National Academy of Sciences.
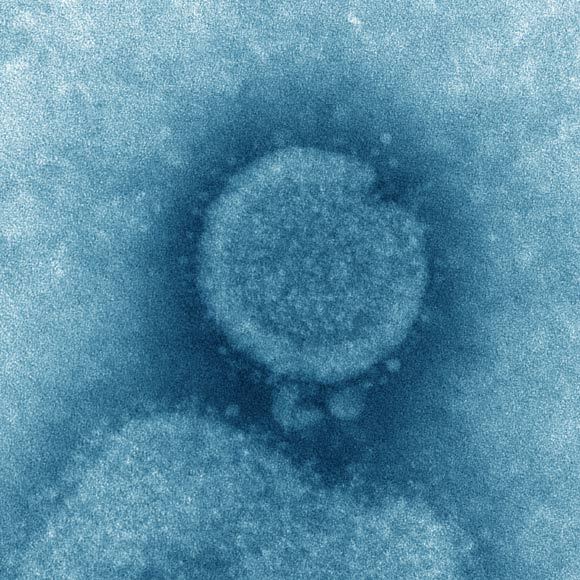
This transmission electron microscopic (TEM) image captured some of the ultrastructural details exhibited by the influenza A (H7N9) virus. Image credit: Cynthia S. Goldsmith & Thomas Rowe, CDC.
Influenza is a persistent threat to public health. Mismatched seasonal influenza vaccines are low efficacy and provide limited protection against circulating strains.
A universal vaccine that can induce broadly cross-protective immunity is urgently needed to eliminate the threats of influenza epidemics and pandemics. Nanotechnology provides a promising approach for developing such vaccines.
To construct nanoparticles for the experimental vaccine, Dr. Wang and co-authors used peptides, compounds consisting of two or more amino acids linked in a chain, because they are much smaller than proteins.
The nanoparticles mimic the biological cues of viruses and initiate danger signals that activate immune responses.
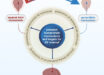
Each double-layered nanoparticle has a core made of peptides from nucleoprotein (NP), an internal influenza protein that has been found to produce cross-protection against influenza virus by inducing T-cell immune responses.
The nanoparticle also has an outside coating made of four peptides from the ectodomain of the influenza A M2 protein (M2e), an evolutionarily conserved region in most human seasonal influenza A viruses and a promising target for universal flu vaccines.
The M2e sequences came from human, swine and avian influenza strains.
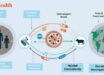
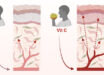
The peptide-only, double-layered nanoparticles, which were delivered by skin vaccination with a dissolvable microneedle patch, induced robust, long-lasting protective immunity and guarded mice against exposure to influenza A virus.
“The adaptive immune system includes B lymphocytes mediating antibody responses and T lymphocytes mediating cellular responses,” Dr. Wang said.
“Our novel nanoparticles trigger immune responses of both immune branches.”
“We have seen the synergistic role of the two branches in providing broad cross-protection against a wide range of diverse influenza virus challenges after vaccination with these layered peptide nanoparticles.”
“The immune protection has also been improved by using a new syringe-free, painless, thermostable and self-applicable microneedle patch.”
The researchers administered the double-layered nanoparticle vaccine to mice using a microneedle patch, which offers advantages over traditional, intramuscular injection, and then exposed them to influenza A virus to see if the vaccine induced protection against the virus.
They found that mice that received the nanoparticle vaccines completely survived various influenza A virus exposures while all mice that received a placebo died within one week.
“These findings will open a new vision for the development of an affordable universal influenza vaccine,” Dr. Wang said.
In future studies, the scientists plan to build upon this double-layered nanoparticle vaccine by adding the inside portion of the influenza virus’ surface protein, which is known as the stalk, to the nanoparticle vaccine coating.
This vaccine approach could also be used to develop vaccines for other pathogens and cancers. The layered, peptide nanoparticles were found to be potent and stable, which indicates potential applications for other peptide-based vaccines and peptide drug delivery.
_____
Lei Deng et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. PNAS, published online July 31, 2018; doi: 10.1073/pnas.1805713115