The new device, developed by researchers in the Department of Chemical Engineering at MIT, is based on passing air through a stack of charged electrochemical plates. It captures carbon dioxide from streams of any concentration, even down to 400 parts per million currently found in the atmosphere, and allows its release into any carrier stream, including 100% carbon dioxide.
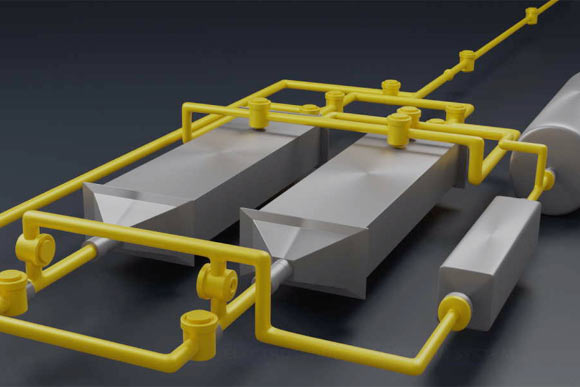
In this diagram of the new system, air entering from top right passes to one of two chambers (the gray rectangular structures) containing battery electrodes that attract carbon dioxide; then the airflow is switched to the other chamber, while the accumulated carbon dioxide in the first chamber is flushed into a separate storage tank (at right); these alternating flows allow for continuous operation of the two-step process. Image credit: Sahag Voskian & T. Alan Hatton, MIT.
The electrochemical device is essentially a large, specialized battery that absorbs carbon dioxide from the air (or other gas stream) passing over its electrodes as it is being charged up, and then releases the gas as it is being discharged.
In operation, the device would simply alternate between charging and discharging, with fresh air or feed gas being blown through the system during the charging cycle, and then the pure, concentrated carbon dioxide being blown out during the discharging.
As the battery charges, an electrochemical reaction takes place at the surface of each of a stack of electrodes. These are coated with a compound called polyanthraquinone, which is composited with carbon nanotubes.
The electrodes have a natural affinity for carbon dioxide and readily react with its molecules in the airstream or feed gas, even when it is present at very low concentrations.
The reverse reaction takes place when the battery is discharged — during which the device can provide part of the power needed for the whole system — and in the process ejects a stream of pure carbon dioxide.
The whole system operates at room temperature and normal air pressure.
“The process this system uses for capturing and releasing carbon dioxide is revolutionary,” said Dr. Sahag Voskian, a postdoctoral researcher at MIT.
“All of this is at ambient conditions — there’s no need for thermal, pressure, or chemical input.”
In the lab, Dr. Voskian and MIT Professor T. Alan Hatton proved the system can withstand at least 7,000 charging-discharging cycles, with a 30% loss in efficiency over that time. They estimate that they can readily improve that to 20,000 to 50,000 cycles.
“The greatest advantage of this technology over most other carbon capture or carbon absorbing technologies is the binary nature of the adsorbent’s affinity to carbon dioxide,” Dr. Voskian said.
“In other words, the electrode material, by its nature, has either a high affinity or no affinity whatsoever, depending on the battery’s state of charging or discharging.”
“Other reactions used for carbon capture require intermediate chemical processing steps or the input of significant energy such as heat, or pressure differences.”
The new device is described in a paper in the journal Energy and Environmental Science.
_____
Sahag Voskian & T. Alan Hatton. Faradaic electro-swing reactive adsorption for CO2 capture. Energy and Environmental Science, published online October 1, 2019; doi: 10.1039/C9EE02412C