Water ice has many crystalline phases, along with a few amorphous structures. The complex structural diagram is important to understand because of the widespread importance of ice. University College London researcher Alexander Rosu-Finsen and his colleagues discovered a medium-density amorphous ice formed by ball milling hexagonal ice at low temperatures. Ball-milling is regularly used to make amorphous materials, but it had never been applied to ice.

A jar with the medium-density amorphous ice inside, with steel balls and liquid nitrogen. Image credit: Rosu-Finsen et al., doi: 10.1126/science.abq2105.
Frozen water can take many forms. There are 20 known common or crystalline phases of water ice and at least two families of amorphous form.
Unlike common ice, whose molecules are regularly arranged in a hexagonal lattice, amorphous forms lack a highly ordered crystalline structure.
Although almost all frozen water on Earth exists as crystalline ice, amorphous ice is likely the most common structure for water in the Universe at large.
In general, amorphous ices are distinguished by their densities, with low-density amorphous ice having a density of 0.94 g/cm3 and high-density amorphous ice forms, which start at 1.13 g/cm3.
However, neither crystalline nor amorphous ices have a form with a density near that of liquid water (1 g/cm3). This density gap is a cornerstone of our current understanding of water.
For the study, Dr. Rosu-Finsen and co-authors used a process called ball milling, vigorously shaking ordinary ice together with steel balls in a jar cooled to 77 K (minus 200 degrees Celsius).
They found that, rather than ending up with small bits of ordinary ice, the process yielded a novel amorphous form of ice that, unlike all other known ices, had the same density (1.06 g/cm3) as liquid water and whose state resembled water in solid form.
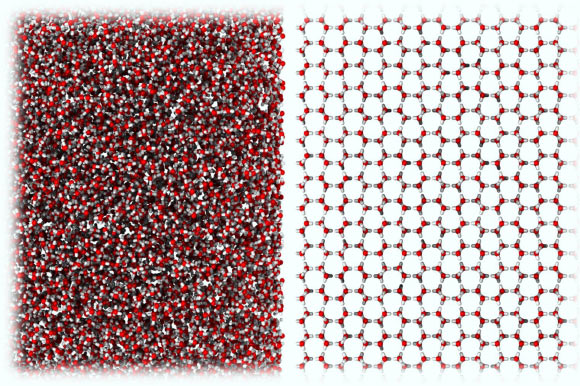
A new form of ice very similar in molecular structure to liquid water (left), compared to ordinary crystalline ice (right). Image credit: Rosu-Finsen et al., doi: 10.1126/science.abq2105.
To understand the process at the molecular scale, they employed computational simulation.
By mimicking the ball-milling procedure via repeated random shearing of crystalline ice, they successfully created a computational model of the medium-density amorphous ice.
“Our discovery of the medium-density amorphous ice raises many questions on the very nature of liquid water and so understanding the new form’s precise atomic structure is very important,” said Dr. Michael Davies, a researcher at University College London and the University of Cambridge.
“We found remarkable similarities between the medium-density amorphous ice and liquid water.”
“The accepted wisdom has been that no ice exists within that density gap,” said University College London’s Professor Christoph Salzmann.
“Our study shows that the density of the medium-density amorphous ice is precisely within this density gap and this finding may have far-reaching consequences for our understanding of liquid water and its many anomalies.”
The discovery of the medium-density amorphous ice gives rise to the question: where might it exist in nature?
Shear forces were discovered to be key to creating the medium-density amorphous ice in the study.
The authors suggest ordinary ice could undergo similar shear forces in the ice moons due to the tidal forces exerted by gas giants such as Jupiter.
Moreover, the medium-density amorphous ice displays one remarkable property that is not found in other forms of ice.
Using calorimetry, the researchers found that when the medium-density amorphous ice recrystallizes to ordinary ice it releases an extraordinary amount of heat.
The heat released from the recrystallization of the medium-density amorphous ice could play a role in activating tectonic motions.
More broadly, this discovery shows water can be a high-energy geophysical material.
“Amorphous ice in general is said to be the most abundant form of water in the Universe,” said Professor Angelos Michaelides, a researcher at University College London and the University of Cambridge.
“The race is now on to understand how much of it is the medium-density amorphous ice and how geophysically active the new form is.”
The discovery is reported in a paper published this week in the journal Science.
_____
Alexander Rosu-Finsen et al. 2023. Medium-density amorphous ice. Science 379 (6631): 474-478; doi: 10.1126/science.abq2105