A team of chemists from Finland and Canada has been able to identify the mechanism that enables some non-metal compounds to mimic the reactivity of their metal-based counterparts.
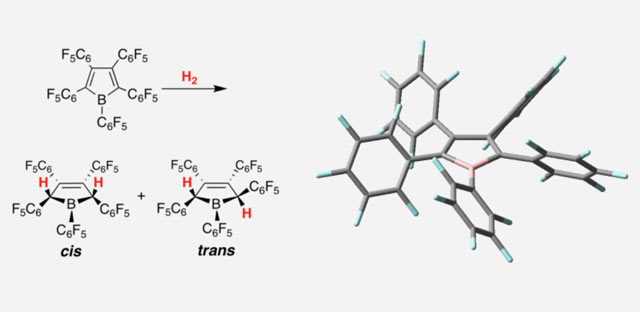
Left: activation of molecular hydrogen with non-metal boroles. Right: molecular structure of a pentaaryl borole (Suomen Akatemia)
“These results could be dubbed a kind of ‘modern alchemy’ in which chemical compounds are made to react in a way totally atypical to them. In alchemy, one of the main goals was to turn the less noble base metals into the more noble ones such as gold,” said Dr Heikki Tuononen of Suomen Akatemia, Finland, senior author of the paper reporting the results in the Journal of the American Chemical Society.
The addition of molecular hydrogen to chemical compounds is one of the most widely used chemical reactions in industries. This reaction typically uses a metal-based catalyst to increase the reaction rate, as otherwise the breakup of the chemical bond in the hydrogen molecule would require significant amount of energy. Since the catalysts used in the process are in general expensive, there has been an ongoing effort to find cheaper and safer hydrogen-activation routes based on non-metal compounds.
“There are only a handful of non-metal compounds known which are able to activate molecular hydrogen in ambient conditions. So the boroles reported in 2010 by Dr. Piers were certainly a welcomed addition to the group! However, at that time, the mechanism of their metal-like reactivity could not be explained,” Dr Tuononen explained.
“The computational results were able to explain why these specific boroles react with molecular hydrogen in a seemingly similar manner to the traditional metal-based compounds even though they contain no metal atoms,” said co-author Dr Virve Karttunen.
The theoretical results obtained by the team show clearly that the reactivity observed for the boroles can be explained with their unique electronic structure. Although the investigated compounds will not function as new catalysts, the results fully support the idea that metal-like reactivity is possible for non-metal compounds and that we have just barely scratched the surface of this research area.
_______
Bibliographic information: Houghton AY et al. 2013. Mechanistic Studies on the Metal Free Activation of Dihydrogen by Antiaromatic Pentarylboroles. J. Am. Chem. Soc., 135, 941-947; doi: 10.1021/ja311842r



![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)



