An international team of researchers, led by Dr. Thomas Pichler from the University of Vienna, has presented a novel method to grow stable, ultra-long linear carbon chains.
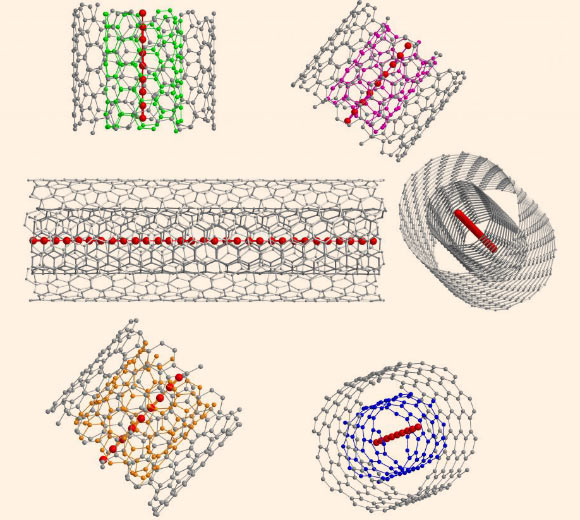
Schematic representation of confined ultra-long acetylenic linear carbon chains inside different double-walled carbon nanotubes. Image credit: Lei Shi / University of Vienna.
Even in its elemental form, the high bond versatility of carbon allows for many different well-known materials, including diamond and graphite. A single layer of graphite, named graphene, can then be rolled or folded into carbon nanotubes or fullerenes, respectively. To date, Nobel prizes have been awarded for both graphene and fullerenes.
Although the existence of carbyne, an infinitely long carbon chain, was proposed in 1885 by Adolf von Baeyer, scientists have not yet been able to synthesize this material.
Von Baeyer even suggested that carbyne (also known as linear acetylenic carbon) would remain elusive as its high reactivity would always lead to its immediate destruction.
Nevertheless, carbon chains of increasing length have been successfully synthesized over the last five decades, with a record of around 100 carbon atoms.
To grow even longer carbon chains – up to 6,000 carbon atoms long – on a bulk scale, Dr. Pichler and his colleagues used the confined space inside a double-walled carbon nanotube as a nano-reactor.
“The direct experimental proof of confined ultra-long linear carbon chains, which are more than an order of magnitude longer than the longest proven chains so far, can be seen as a promising step towards the final goal of unraveling the ‘holy grail’ of carbon allotropes, carbyne,” said team member Lei Shi, from the Faculty of Physics at the University of Vienna.
“Carbyne is very stable inside double-walled carbon nanotubes,” the scientists said. “This property is crucial for its eventual application in future materials and devices.”
“According to theoretical models, carbyne’s mechanical properties exceed all known materials, outperforming both graphene and diamond.”
“Carbyne’s electrical properties suggest novel nanoelectronic applications in quantum spin transport and magnetic semiconductors.”
The results were published online April 4, 2016 in the journal Nature Materials (arXiv.org preprint).
_____
Lei Shi et al. Confined linear carbon chains as a route to bulk carbine. Nature Materials, published online April 4, 2016; doi: 10.1038/nmat4617





![Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.](https://cdn.sci.news/images/2025/08/image_14141-Cyclo-48-Carbon-104x75.jpg)

