The dissemination of antibiotic resistance genes poses a severe and global threat to public health. A new comprehensive review by Hohai University scientists explores the evolutionary origins, the ecological drivers underlying the proliferation and dissemination of antibiotic resistance genes, and their far-reaching environmental implications.
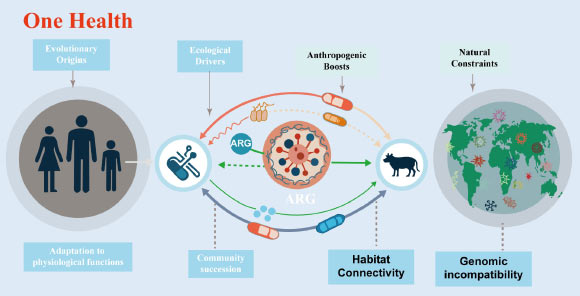
The evolution of antibiotic resistance genes is linked to intrinsic physiological roles and ecological divisions. Image credit: Xu et al., doi: 10.48130/biocontam-0025-0014.
Antibiotic resistance genes have emerged as one of the most pressing global threats to public health, with their dissemination spanning the interconnected compartments of humans, animals, and the environment.
They have been detected in some of the most extreme and undisturbed environments on Earth, including the depths of the Mariana Trench, pristine Alaskan soil, and 30,000-year-old permafrost sediments, settings entirely untouched by anthropogenic antibiotic exposure.
This broad distribution confirms a critical truth: these bacterial species evolved the ability to tolerate antibiotics millions of years before the production of antibiotics for clinical and agricultural applications.
“Antibiotic resistance did not begin with modern medicine,” said Dr. Guoxiang You, corresponding author of the study.
“Many resistance genes originally evolved to help bacteria survive environmental stresses, long before humans discovered antibiotics.”
“The real danger today comes from how human activities are breaking down natural barriers and allowing these genes to spread into pathogens.”
“Many resistance genes are derived from ordinary bacterial genes with essential physiological roles, such as pumping out toxic substances or transporting nutrients,” the researchers said.
“Over evolutionary time, these genes gained the ability to defend against antibiotics as a secondary function.”
In undisturbed ecosystems like soils, lakes, and remote environments, most resistance genes remain locked within specific microbial communities and pose little risk to human health.
“A key reason for this containment is genomic incompatibility,” they added.
“Bacteria that are genetically very different often cannot easily exchange and use resistance genes.”
“This natural mismatch acts as a biological firewall, limiting the spread of resistance across species and habitats.”
“However, human activity is weakening this firewall.”
In the review, the authors highlights how agriculture, wastewater discharge, urbanization, and global trade increase connectivity between environments that were once separate.
Antibiotics used in medicine and livestock create strong selection pressures, while manure application, wastewater reuse, and environmental pollution bring together bacteria from soil, animals, and humans.
These conditions make it easier for resistance genes to jump into disease causing microbes.
“Human driven habitat connectivity changes everything,” said Dr. Yi Xu, first author of the study.
“When bacteria from different environments are repeatedly brought into contact under antibiotic pressure, resistance genes that were once harmless can become a serious public health threat.”
“Wastewater treatment plants are identified as critical hotspots, where high bacterial densities and residual antibiotics promote gene exchange.”
“Agricultural soils fertilized with manure can also act as bridges, allowing resistance genes to move from livestock into environmental bacteria and eventually back to humans through food, water, or direct contact.”
Importantly, the scientists stress that not all resistance genes are equally dangerous.
High abundance in the environment does not automatically mean high risk.
Understanding which genes are mobile, compatible with human pathogens, and linked to disease is essential for effective monitoring and control.
The researchers call for ecosystem-centered strategies to combat antibiotic resistance.
These include reducing unnecessary antibiotic use, improving wastewater treatment technologies, managing manure and sludge more carefully, and protecting relatively pristine ecosystems that serve as baselines for natural resistance levels.
“Antibiotic resistance is not just a medical issue,” Dr. You said.
“It is an ecological problem rooted in how we interact with the environment.”
“Protecting antibiotics for future generations requires protecting ecosystem integrity today.”
“By integrating evolutionary biology, microbial ecology, and environmental science, a One Health approach offers the most realistic path forward in addressing one of the greatest global health challenges of our time.”
The review was published online on December 5, 2025 in the journal Biocontaminant.
_____
Yi Xu et al. 2025. Evolutionary origins, ecological drivers, and environmental implications of antibiotic resistance genes proliferation and dissemination: a ‘One Health’ perspective. Biocontaminant 1: e014; doi: 10.48130/biocontam-0025-0014







