According to a new research headed by virologist Dr Edward Campbell from Loyola University Chicago, a protein called alpha-synuclein, known to be a key player in the development of Parkinson’s disease, is able to enter and harm cells in the same way that viruses do.
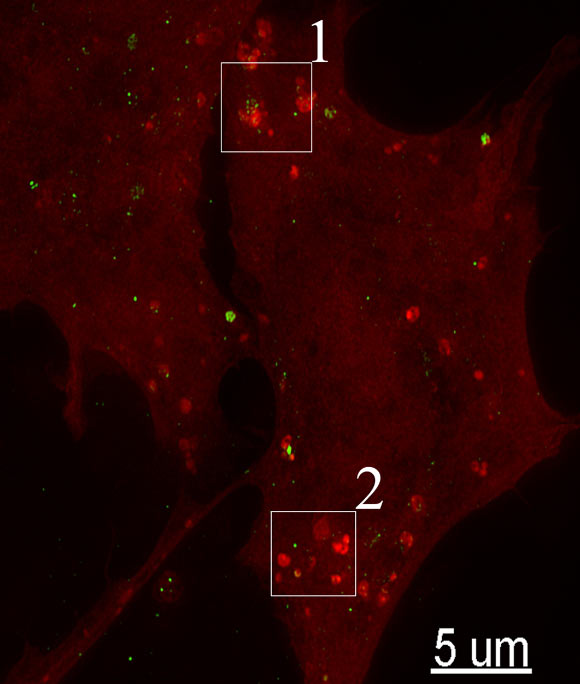
Structured illumination imaging of rat neuronal cells (Freeman D et al)
Alpha-synuclein plays a role in the normal functioning of healthy neurons. But in Parkinson’s disease patients, the protein turns bad, aggregating into clumps that lead to the death of neurons in the area of the brain responsible for motor control. Previous studies have shown that these protein aggregates can enter and harm cells.
Dr Campbell’s team showed how alpha-synuclein can bust out of lysosomes, small structures that collectively serve as the cell’s digestive system. The rupture of these bubble-like structures, known as vesicles, releases enzymes that are toxic to the rest of the cell.
“The finding eventually could lead to the development of new therapies to delay the onset of Parkinson’s disease or halt or slow its progression,” explained Dr Campbell and his colleagues, who reported their results in the open access journal PLoS ONE.
“The release of lysosomal enzymes is sensed as a ‘danger signal’ by cells, since similar ruptures are often induced by invading bacteria or viruses,” said study co-author Dr Chris Wiethoff, also from Loyola University Chicago.
“Lysosomes are often described as ‘suicide bags’ because when they are ruptured by viruses or bacteria, they induce oxidative stress that often leads to the death of the affected cell.”
“In a viral or bacterial infection, the deaths of such infected cells may overall be a good thing for the infected individual. But in Parkinson’s disease, this same protective mechanism may lead to the death of neurons and enhance the spread of alpha-synuclein between cells in the brain,” Dr Campbell said.
“This might explain the progressive nature of Parkinson’s disease. More affected cells leads to the spread of more toxic alpha-synuclein aggregates in the brain. This is very similar to what happens in a spreading viral infection.”
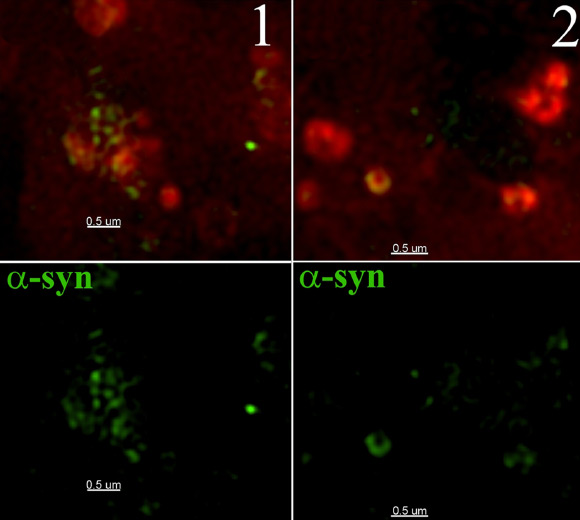
Structured illumination imaging reveals localization of alpha-synuclein in rat neuronal cells (Freeman D et al)
“These studies need to be followed up and confirmed in other models of Parkinson’s disease. Using cultured cells, we have made some exciting observations. However, we need to understand how lysosomal rupture is affecting disease progression in animal models of Parkinson’s disease and, ultimately, the brains of people affected by Parkinson’s disease. Can we interfere with the ability of alpha-synuclein to rupture lysosomes in these settings? And will that have a positive effect on disease progression? These are the questions we are excited to be asking next.”
“The study is an important finding by a group of investigators who are beginning to make their impact in the field of Parkinson’s disease. This paper adds to the growing concept that alpha-synuclein, a main culprit in the cause of Parkinson’s disease, can transfer from cell to cell. This paper elegantly puts a mechanism behind such a transfer. The findings will help shape the direction of Parkinson’s disease research for years to come,” concluded Prof Jeffrey Kordower from Rush University Medical Center, who didn’t take part in the study.
______
Bibliographic information: Freeman D et al. 2013. Alpha-Synuclein Induces Lysosomal Rupture and Cathepsin Dependent Reactive Oxygen Species Following Endocytosis. PLoS ONE 8 (4): e62143; doi: 10.1371/journal.pone.0062143







